Key takeaways:
~ Arsenic naturally occurs in well water in some areas, and it is a contaminant found in the soil in other areas.
~ Rice grown in areas with arsenic contamination can absorb high levels of it.
~ The body can detoxify arsenic using specific enzymes and pathways.
~ Genetic variants, though, can impact how well you detoxify arsenic.
This article covers the pathways the body uses to get rid of arsenic and includes information on genetic variants that may impair the detoxification of arsenic.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.Arsenic, detoxification pathways, and genetics:
Let’s start with a bit of background science: what exactly is arsenic, and how do we get rid of it?
Arsenic is a naturally occurring semi-metal metalloid and is usually found as a compound with other minerals. Arsenic exposure occurs via well water, in certain foods, and through breathing (industrial pollution).
Your body has built-in pathways for removing arsenic. It is a naturally occurring toxin, and animals have detoxification pathways for getting rid of it – up to a point… Arsenic is, of course, deadly at certain levels. Your genes impact how well your body gets rid of arsenic.
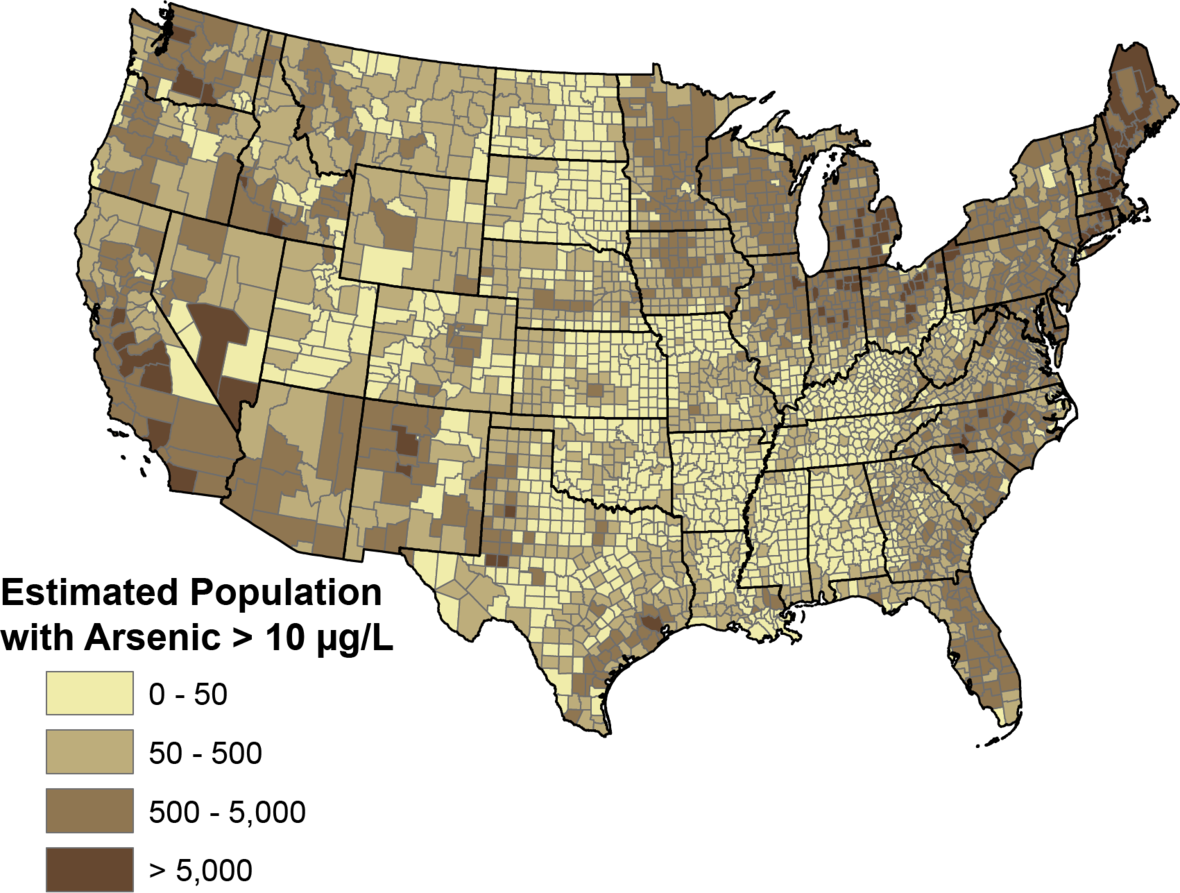
In the US, about 7% of water wells contain arsenic at 10 µg/L levels. The EPA lowered the target level of arsenic from 50 µg/L (50 ppb) to 10 µg/L (10 ppb) in 2006.[ref]
If you live in the US, here is a USGS map showing counties with higher exposure to arsenic in the well water.
Arsenic can also be found in foods grown in contaminated soil or water. For example, depending on where rice is grown affects its varying amounts of arsenic. Organic brown rice grown in Australia contains about the maximum WHO recommended daily limit of arsenic. It compares with rice from India, which had about a quarter of that amount.[ref]
In fact, people eating a gluten-free diet in the US are, on average, exposed to a lot more arsenic from rice. A study showed that people eating gluten-free had almost double the arsenic concentration in their urine. This is due to gluten-free bread, pasta, and pretzels being made from rice flour.[ref]
The good news is that your body has built-in ways of getting rid of arsenic.
How does your body get rid of arsenic?
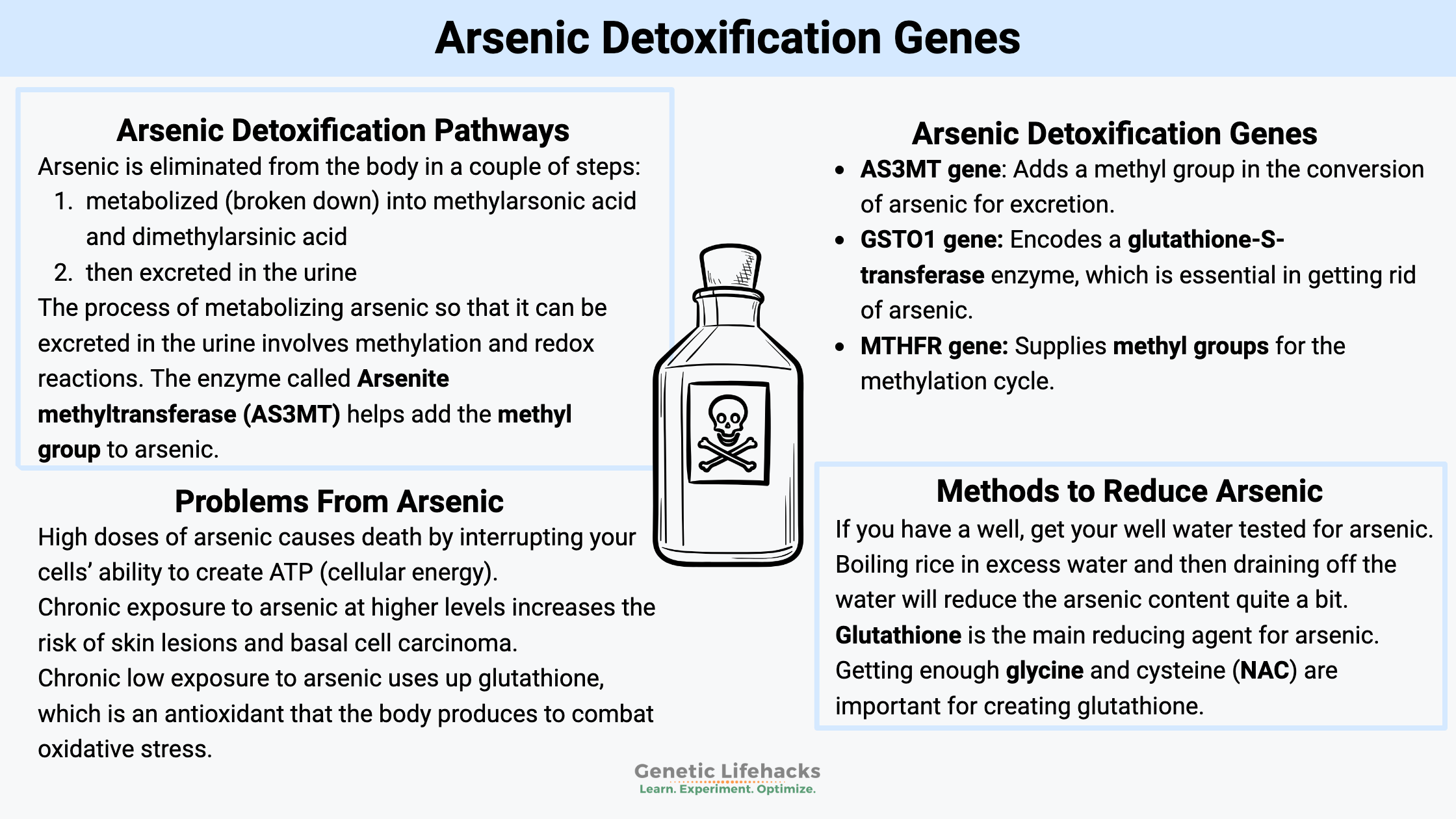
Arsenic is eliminated from the body in a couple of steps:
- metabolized (broken down) into methylarsonic acid and dimethylarsinic acid
- then excreted in the urine
Getting a little more in-depth:
The process of metabolizing arsenic so that it can be excreted in the urine involves methylation and redox reactions. Methylation refers to adding a methyl (CH3) group to the molecule. And the redox reaction involves glutathione as the reducing agent. The key is that once this detoxification starts, the process needs to continue to the point of excretion. This is because some of the intermediates that are formed are even more toxic than the original arsenic.[ref]
The enzyme called Arsenite methyltransferase (AS3MT) helps add the methyl group to arsenic. Because it uses a methyl group, the detoxification process depends on the body having sufficient methyl groups available.
Your cells use glutathione for the redox reactions that occur during arsenic metabolism. Thus, adequate glutathione is also necessary here.
How does arsenic cause problems in the body?
Arsenic as poison has an interesting history.
Arsenic trioxide was called “succession” or “inheritance powder”, and was historically used in Italy and France to poison several leaders. The Borgias family in Italy is famous for using a little arsenic in wine to gain wealth and power.[ref]
In the Victorian era, a pale complexion was all the rage. Women would mix vinegar, chalk, and arsenic trioxide together and eat it to become pale. Essentially, they were poisoning themselves slightly in the name of beauty.
Arsenic poisoning from high doses:
A high dose of arsenic causes death by interrupting your cells’ ability to create ATP (cellular energy).
Arsenic in different forms can affect either glycolysis (turning glucose into energy) or the Krebs cycle (producing ATP/energy in the mitochondria).
As you can imagine, reducing the production of ATP isn’t a great idea. If you reduce ATP enough, cell death occurs.
Skin lesions and skin cancer:
Chronic exposure to arsenic at higher levels (>100 μg/L in drinking water) increases the risk of skin lesions[ref] and basal cell carcinoma, a type of skin cancer.[ref]
At higher levels, arsenic increases the risk of skin lesions or cancer for everyone. But some people may be at an increased risk with only moderate levels of arsenic exposure.
Genetic variants associated with less efficient arsenic methylation are linked to an increase in the risk of basal cell carcinoma (skin cancer).[ref]
You may wonder if showering or bathing in arsenic water causes skin cancer. That doesn’t seem to be the case. Arsenic is not well absorbed transdermally; instead, the consumed (food, water) arsenic concentrates in skin, hair, and nails.[ref]
Chronic low exposure to arsenic uses up glutathione
While lower amounts of arsenic in drinking water (<50 μg/L) may not cause death or cancer, there can be long-term consequences.
Constantly detoxifying arsenic uses a lot of glutathione. Glutathione is an antioxidant that the body produces to combat oxidative stress in cells and to detoxify several different substances -including arsenic.[ref]
When arsenic is methylated in the first step of detoxification, the intermediary compounds produced are actually more toxic than the original inorganic arsenic. If the cells don’t have enough glutathione or methyl groups available to complete the detoxification and excretion, the toxic metabolites can hang out in the body for too long. It is one way that arsenic increases the risk of cancer.[ref]
Mitochondrial function:
Arsenic can also increase cellular oxidative stress in several ways, such as impacting mitochondrial energy production. Nrf2 is one way that cells can combat oxidative stress. Indeed, research shows that arsenic exposure upregulates the Nrf2 pathway, and Nrf2 protects the cells against arsenic.[ref]
Heart disease:
Higher exposure to arsenic over 10 years in community well water is associated with an increased risk of heart disease. The large epidemiological study involved almost 100,000 women and showed an increased relative risk of heart disease of more than 20%.[ref]
Breast cancer:
Exposure to arsenic in drinking water is also associated with an increased relative risk of breast cancer of 10-15%, depending on the study. While this is a very small increase in relative risk, when stratified by genetic variants in the AS3MT gene, the risk jumps to 2 to 3-fold increased risk, which is very significant.[ref][ref] In addition, higher levels of arsenic (through drinking water, rice consumption, etc.) are also linked to increased breast cancer risk. A large Canadian study showed that women with higher urinary arsenic levels were at a 2-fold increased risk of breast cancer.[ref]
We are all unique in how well we detoxify arsenic, so let’s look at how your genes influence all these different steps in arsenic detoxification.
Arsenic Detoxification Genotype Report:
Lifehacks: Ways to mitigate exposure and detoxify arsenic
Test your well water:
The biggest source of arsenic exposure for most people is drinking well water that has high levels. Water testing is relatively inexpensive and will let you know your exposure. If your well water comes back higher in arsenic, consider remediating with a reverse osmosis filter system.
Filters to get rid of arsenic:
ConsumerLab.com has tested a bunch of different pitcher-type water filters for their ability to remove arsenic from the water. The ZeroWater, Travel Berkey, and Pur ’20 filters removed about 90% of arsenic. Other commonly used filter pitchers didn’t remove much arsenic at all.[ref]
Cooking methods to reduce arsenic:
Rice is one of the biggest sources of dietary arsenic since it takes up and stores arsenic from the soil and groundwater. Rice from the southern US is especially high in arsenic, on average, due to residual arsenic in the soils from old pesticides used on cotton.
There are two ways of preparing rice that can decrease the arsenic content:
- Boiling rice in excess water (like you do pasta) and then draining off the water will reduce the arsenic content quite a bit.[ref]
- Soak or even parboil the rice first (discarding the soaking water) and then cook normally to decrease arsenic.[ref]
Of note here: if you cook rice in water that contains arsenic, the rice will absorb the arsenic from the water.[ref]
ConsumerLab.com has also tested rice for arsenic levels and ranked them based on cost per gram of rice and arsenic. They found that Trader Joe’s Jasmine rice was the second least expensive ($0.11 cents per serving) and also contained the second least amount of arsenic (0.05 mcg/g). Out of all of the different types of rice, ConsumerLab found to be the ‘safest’ (>.01 mcg/g of arsenic) were seven white rices and one brown rice. No long-grain or wild rices were found to be safe for infant consumption.
Folate:
Get your methylation cycle on track. The methylation cycle is at the heart of many processes in the body, including arsenic detoxification. If you carry the MTHFR C677T variant, it is essential to get enough folate daily. Higher levels of folate intake reduce the risk of arsenic toxicity.[ref]
Foods high in folate include dark leafy green vegetables, lentils, and beef liver. Additionally, methyl folate supplements are available. The recommended daily intake of folate is 400 ug. (Check your COMT variants before supplementing with high doses of methyl supplements).
Lifestyle factors:
Smoking and drinking both decrease your ability to get rid of arsenic.[ref]
Supplements to consider for arsenic detoxification:
Related Articles and Topics:
Nrf2 Pathway: Increasing the body’s ability to get rid of toxins
The Nrf2 (Nuclear factor erythroid 2–related factor) signaling pathway regulates the expression of antioxidants and phase II detoxification enzymes. It is a fundamental pathway important in how well your body functions. Your genetic variants in the NFE2L2 gene impact this NRF2 pathway.
Detoxification: Phase I and Phase II Metabolism
Phase I and Phase II of detoxification rely on specific genes. Variants in these pathways impact your reaction to toxins, chemicals, and medications.
MTHFR – Beyond C677T and A1298C
The MTHFR C677T and A1298C variants get a lot of press, but they do not give the whole picture of the MTHFR gene. Additional variants are impacting the functionality of the enzyme.
GSTs: glutathione-S-transferase enzymes for detoxifying environmental toxins
Exposure to many different man-made chemical compounds occurs every day, and our exposure to new toxicants exceeds what our ancestors experienced. Several common GST variants decrease the function of the GST enzymes.
References:
Amorós, Rubén, et al. “Selenium Status during Pregnancy: Influential Factors and Effects on Neuropsychological Development among Spanish Infants.” The Science of the Total Environment, vol. 610–611, Jan. 2018, pp. 741–49. PubMed, https://doi.org/10.1016/j.scitotenv.2017.08.042.
Argos, Maria, et al. “A Prospective Study of Arsenic Exposure from Drinking Water and Incidence of Skin Lesions in Bangladesh.” American Journal of Epidemiology, vol. 174, no. 2, July 2011, pp. 185–94. PubMed, https://doi.org/10.1093/aje/kwr062.
Arsenic: A Murderous History | Dartmouth Toxic Metals. https://sites.dartmouth.edu/toxmetal/arsenic/arsenic-a-murderous-history/. Accessed 18 July 2022.
Choudhury, Sreetama, et al. “Pomegranate Protects against Arsenic-Induced P53-Dependent ROS-Mediated Inflammation and Apoptosis in Liver Cells.” The Journal of Nutritional Biochemistry, vol. 38, Dec. 2016, pp. 25–40. PubMed, https://doi.org/10.1016/j.jnutbio.2016.09.001.
Engström, Karin S., et al. “Genetic Variation in Arsenic (+3 Oxidation State) Methyltransferase ( AS3MT ), Arsenic Metabolism and Risk of Basal Cell Carcinoma in a E Uropean Population.” Environmental and Molecular Mutagenesis, vol. 56, no. 1, Jan. 2015, pp. 60–69. DOI.org (Crossref), https://doi.org/10.1002/em.21896.
García-Alvarado, Francisco Javier, et al. “[Polymorphisms of the Arsenite Methyltransferase (As3MT) gene and urinary efficiency of arsenic metabolism in a population in northern Mexico].” Revista Peruana De Medicina Experimental Y Salud Publica, vol. 35, no. 1, Mar. 2018, pp. 72–76. PubMed, https://doi.org/10.17843/rpmesp.2018.351.3565.
Hu, Yuxin, et al. “The Role of Reactive Oxygen Species in Arsenic Toxicity.” Biomolecules, vol. 10, no. 2, Feb. 2020, p. 240. PubMed Central, https://doi.org/10.3390/biom10020240.
Kile, Molly L., and Alayne G. Ronnenberg. “Can Folate Intake Reduce Arsenic Toxicity?” Nutrition Reviews, vol. 66, no. 6, June 2008, pp. 349–53. PubMed Central, https://doi.org/10.1111/j.1753-4887.2008.00043.x.
Luo, Lanrong, et al. “Association between Arsenic Metabolism Gene Polymorphisms and Arsenic-Induced Skin Lesions in Individuals Exposed to High-Dose Inorganic Arsenic in Northwest China.” Scientific Reports, vol. 8, Jan. 2018, p. 413. PubMed Central, https://doi.org/10.1038/s41598-017-18925-3.
Martinez, Victor D., et al. “Induction of Human Squamous Cell-Type Carcinomas by Arsenic.” Journal of Skin Cancer, vol. 2011, 2011, p. 454157. PubMed Central, https://doi.org/10.1155/2011/454157.
Miraghaee, Seyyed Shahram, et al. “Assessment of GSTO1 (A140D) and GSTO2 (N142D) Gene Polymorphisms in Iranian Women with Polycystic Ovarian Syndrome.” Reports of Biochemistry & Molecular Biology, vol. 9, no. 1, Apr. 2020, pp. 8–13. PubMed Central, https://doi.org/10.29252/rbmb.9.1.8.
Rahman, M. Azizur, et al. “Arsenic Speciation in Australian-Grown and Imported Rice on Sale in Australia: Implications for Human Health Risk.” Journal of Agricultural and Food Chemistry, vol. 62, no. 25, June 2014, pp. 6016–24. DOI.org (Crossref), https://doi.org/10.1021/jf501077w.
Rodrigues, Ema G., et al. “GSTO and AS3MT Genetic Polymorphisms and Differences in Urinary Arsenic Concentrations among Residents in Bangladesh.” Biomarkers, vol. 17, no. 3, May 2012, pp. 240–47. PubMed Central, https://doi.org/10.3109/1354750X.2012.658863.
Sage, Adam P., et al. “Oncogenomic Disruptions in Arsenic-Induced Carcinogenesis.” Oncotarget, vol. 8, no. 15, Feb. 2017, pp. 25736–55. PubMed Central, https://doi.org/10.18632/oncotarget.15106.
Shen, Hui, et al. “Factors Affecting Arsenic Methylation in Arsenic-Exposed Humans: A Systematic Review and Meta-Analysis.” International Journal of Environmental Research and Public Health, vol. 13, no. 2, Feb. 2016, p. 205. PubMed Central, https://doi.org/10.3390/ijerph13020205.
Skröder, Helena, et al. “Associations between Methylated Metabolites of Arsenic and Selenium in Urine of Pregnant Bangladeshi Women and Interactions between the Main Genes Involved.” Environmental Health Perspectives, vol. 126, no. 2, Feb. 2018, p. 027001. PubMed Central, https://doi.org/10.1289/EHP1912.
Stajnko, Anja, et al. “Arsenic Metabolites; Selenium; and AS3MT, MTHFR, AQP4, AQP9, SELENOP, INMT, and MT2A Polymorphisms in Croatian-Slovenian Population from PHIME-CROME Study.” Environmental Research, vol. 170, Mar. 2019, pp. 301–19. PubMed, https://doi.org/10.1016/j.envres.2018.11.045.
US EPA, OW. Chemical Contaminant Rules. 13 Oct. 2015, https://www.epa.gov/dwreginfo/chemical-contaminant-rules.
Valenzuela, Olga L., et al. “Association of AS3MT Polymorphisms and the Risk of Premalignant Arsenic Skin Lesions.” Toxicology and Applied Pharmacology, vol. 239, no. 2, Sept. 2009, pp. 200–07. PubMed, https://doi.org/10.1016/j.taap.2009.06.007.
Wan, Lin, et al. “Methylenetetrahydrofolate Reductase and Psychiatric Diseases.” Translational Psychiatry, vol. 8, no. 1, Nov. 2018, pp. 1–12. www.nature.com, https://doi.org/10.1038/s41398-018-0276-6.
Wlodarczyk, Bogdan J., et al. “Mthfr Gene Ablation Enhances Susceptibility to Arsenic Prenatal Toxicity.” Toxicology and Applied Pharmacology, vol. 275, no. 1, Feb. 2014, pp. 22–27. PubMed Central, https://doi.org/10.1016/j.taap.2013.12.014.