Key takeaways:
~ Atrial fibrillation is an irregular heartbeat in which the upper chambers of the heart have an irregular rhythm.
~ Structural changes in the heart muscle or changes in ion channels can increase the risk of atrial fibrillation.
~ Failure to resolve inflammation can lead to changes in heart tissue that make it susceptible to atrial fibrillation.
~ Genetic variants are strongly associated with an increased risk of AFib, especially when combined with inflammation or a triggering event.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What happens in the heart when you have AFib?
Atrial fibrillation – often called AFib or a-fib – is an irregular or quivering heartbeat. It occurs when the upper chambers of the heart, the atria, start to beat out of sync with the lower chambers, the ventricles. This can be something that happens periodically for short periods of time, or it can be chronic.
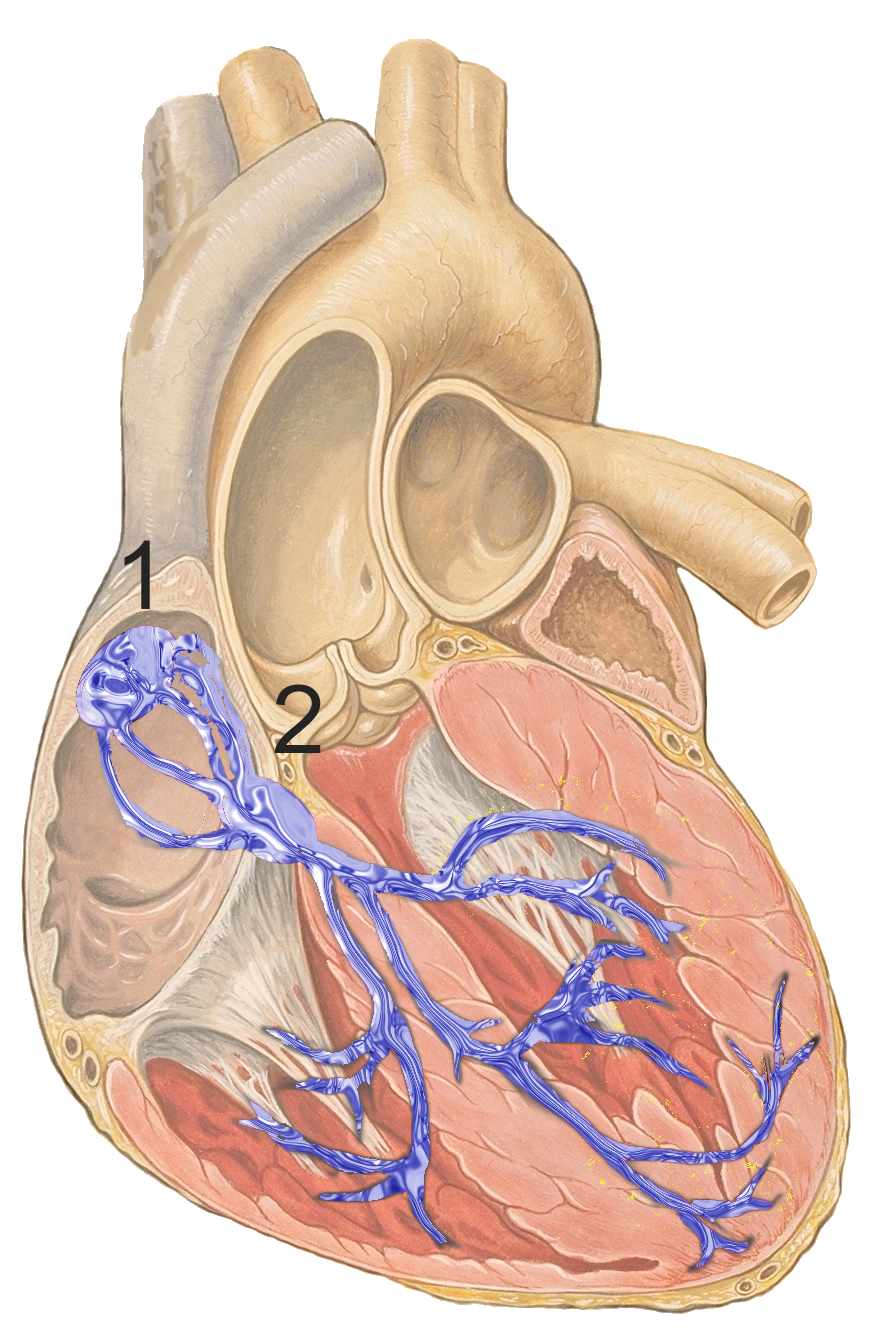
Your normal heartbeat is controlled by an electrical signal that starts at the SA (sinoatrial) node, causes the upper chambers to contract, and then travels down the heart to cause the lower chambers to contract.
In A-Fib, an abnormal electrical signal in the atrium overwhelms the signal from the SA node. This causes the atrium to beat irregularly.[ref]
When the atria contract rapidly and in a disorganized manner, blood doesn’t move as effectively into the lower chambers, the ventricles. This can cause an irregular ventricular response, either slower or faster than normal.
Why is AFib a big deal?
People with atrial fibrillation are at an increased risk of blood clots. Blood can pool in the atrium, causing a blood clot to travel through the veins and eventually cause a blockage. This means that people with chronic atrial fibrillation are at an increased risk of stroke.
For this reason, most people with chronic atrial fibrillation take blood thinners to prevent clots.
Risk Factors for Atrial Fibrillation:
General risk factors for AFib include:[ref][ref]
- age
- coronary artery disease, heart failure
- mitral valve disease
- high blood pressure
- sick sinus syndrome
- hyperthyroidism
- alcoholism, smoking, stimulant drugs
- certain medications
- viral infections
- diabetes, metabolic syndrome
- COPD, sleep apnea, air pollution
- heart surgery
These risk factors can influence the development of AFib in different ways. Let’s look at some examples:
Hypertension changes cardiac muscle:
Someone who has had very high blood pressure for a long time may have an enlarged left ventricle. This physically changes the heart muscle tissue and the way the signal for the heartbeat travels through it.
Inflammation changes cardiac nerve and muscle structure:
Chronic inflammation is also thought to play a causal role in atrial fibrillation. People with rheumatoid arthritis or psoriasis are at a 40% increased relative risk of atrial fibrillation. Both of these autoimmune diseases cause inflammation as well as abnormalities in the way that the electrical activation occurs in the atrium. Similarly, lupus, systemic sclerosis, IBD, and ankylosing spondylitis are also linked to an increased risk of AF due to structural or functional changes in the atrium. [ref]
Exposure to pollution increases inflammation:
Smoking, air pollution, vaping, and excessive alcohol use are all risk factors for AFib.[ref] All can increase oxidative stress in the cardiac muscle.
Inflammation increases in aging leading to structural changes:
Aging is another cause of chronically elevated inflammatory cytokines, which can lead to fibrosis of the heart tissue. As we age, there is an increase in senescent cells, which release inflammatory cytokines and affect surrounding tissue. This is likely why the risk of AFib increases significantly by the age of 80.
Sleep apnea -> increased blood pressure, inflammation:
Sleep apnea also increases the risk of cardiac dysfunction, including atrial fibrillation. Sleep apnea causes periods of low oxygen and high CO2, which causes increased blood pressure and oxidative stress.[ref]
Obesity -> increased inflammation:
Obesity is also a risk factor for AFib. Recent research showed that: “atrial NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmogenesis”. NLRP3 is a gene that encodes the protein cryopyrin. It is a receptor protein that detects products of damaged cells and then sends a signal for inflammation to occur. This study showed that obese mice with the NLRP3 gene knocked out were not susceptible to AFib being induced, while normal obese mice were. The lack of NLRP3 prevented the electrical remodeling and the fibrosis in the heart.[ref]
Related article: NLRP3 genetic variants
A picture emerges here: things that cause systemic inflammation can lead to structural changes to the heart that interfere with good nerve conduction.
But why does inflammation persist?
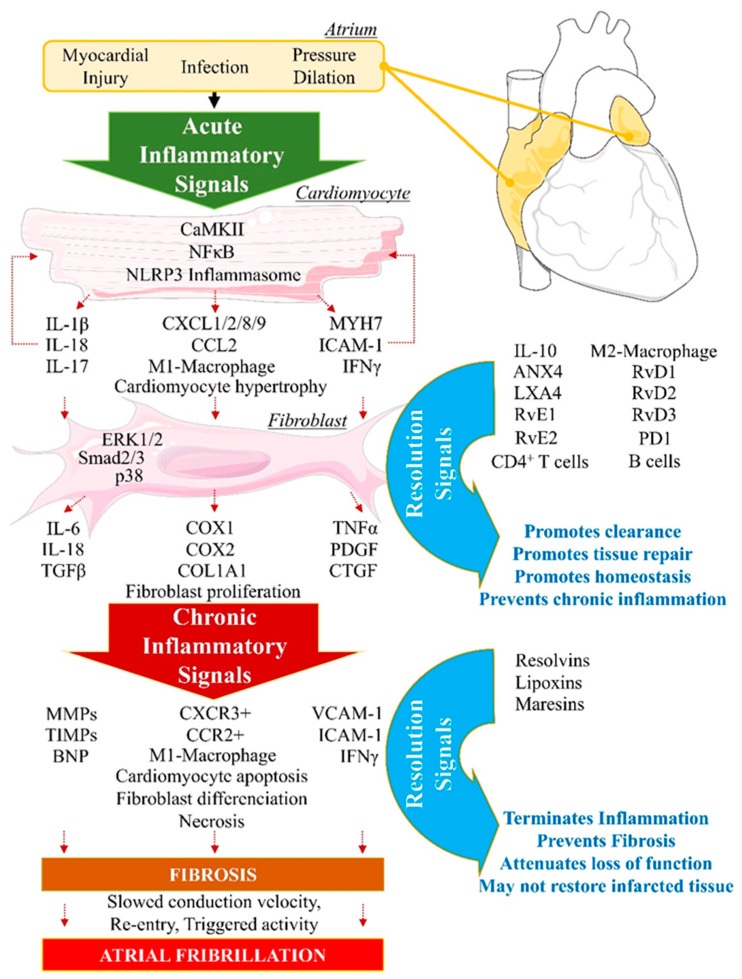
Unresolved Inflammation: Longterm Structural Changes
Ion channel problems or structural issues that increase the risk of Afib are often due to genetic variants (more on this in a minute). But not everyone with genetic risk factors will get AF.
The other half of the picture may be unresolved inflammation. Note that I say “may”. This is new research and there is more to learn, but I wanted to highlight it as a likely cause for many people.
Myocarditis, viral infection, very high blood pressure, cigarette smoking, air pollution, obesity, chronic autoimmune conditions… all of these cause increased inflammatory cytokines in the heart muscle.
If the initial inflammatory signals aren’t resolved, fibrosis can occur in the heart muscle tissue. This remodeling of the heart muscle can be a cause of AF by changing the way the signal is conducted or changing the initiation of the signal from the autonomic nervous system.[ref]
How is inflammation resolved?
The resolution of inflammation is an active process that occurs in parallel with the initial inflammatory event. The key here is that the resolution of inflammation relies on specialized pro-resolving lipid mediators (SPMs) derived from omega-3 fatty acids, DHA and EPA. The specific lipid mediators involved in the resolution of inflammation are resolvins, lipoxins, and maresins. These lipid mediators are signaling molecules. They signal the “off switch” for inflammatory cytokines as well as the “on switch” for tissue healing processes and return to homeostasis.[ref]
Without returning the tissue to normal (without pro-resolving mediators), the heart tissue can be altered, or remodeled, into a substrate that is more likely to cause dysregulation of the heartbeat. (More on increasing pro-resolving lipid mediators in the Lifehacks section)
Related article: Specialized pro-resolving mediators and the resolution of inflammation
When AFib continues to happen…
Let’s define a few terms, first:
- Lone AFib is a term used to describe the onset of atrial fibrillation without the usual risk factors, such as significant heart disease or being born with a congenital heart defect.
- Persistent AFib is defined as episodes lasting longer than one week.
- Permanent AFib is defined as episodes lasting longer than one year.
People with persistent and permanent AFib have higher levels of inflammatory markers including IL-1B, IL-6, TNF-alpha, IL-8, C-reactive protein, and MPO (myeloperoxidase).[ref]
To get a little more specific, let’s look at TNF-alpha as an example. TNF-alpha is an inflammatory cytokine produced in response to pathogens and toxins. TNF receptors are expressed on cardiac myocytes (heart muscle) and endothelial cells that line blood vessels.
Increased TNF-alpha signaling has been shown to “promote atrial electrical, structural, and contractile remodeling, all of which are hallmarks of the molecular pathophysiology of AF.” In addition, elevated TNF-alpha levels have been shown in animal studies to promote atrial fibrosis, which slows and interrupts the conduction of the heartbeat signal.[ref]
Related article: Genetic variants that increase TNF-alpha
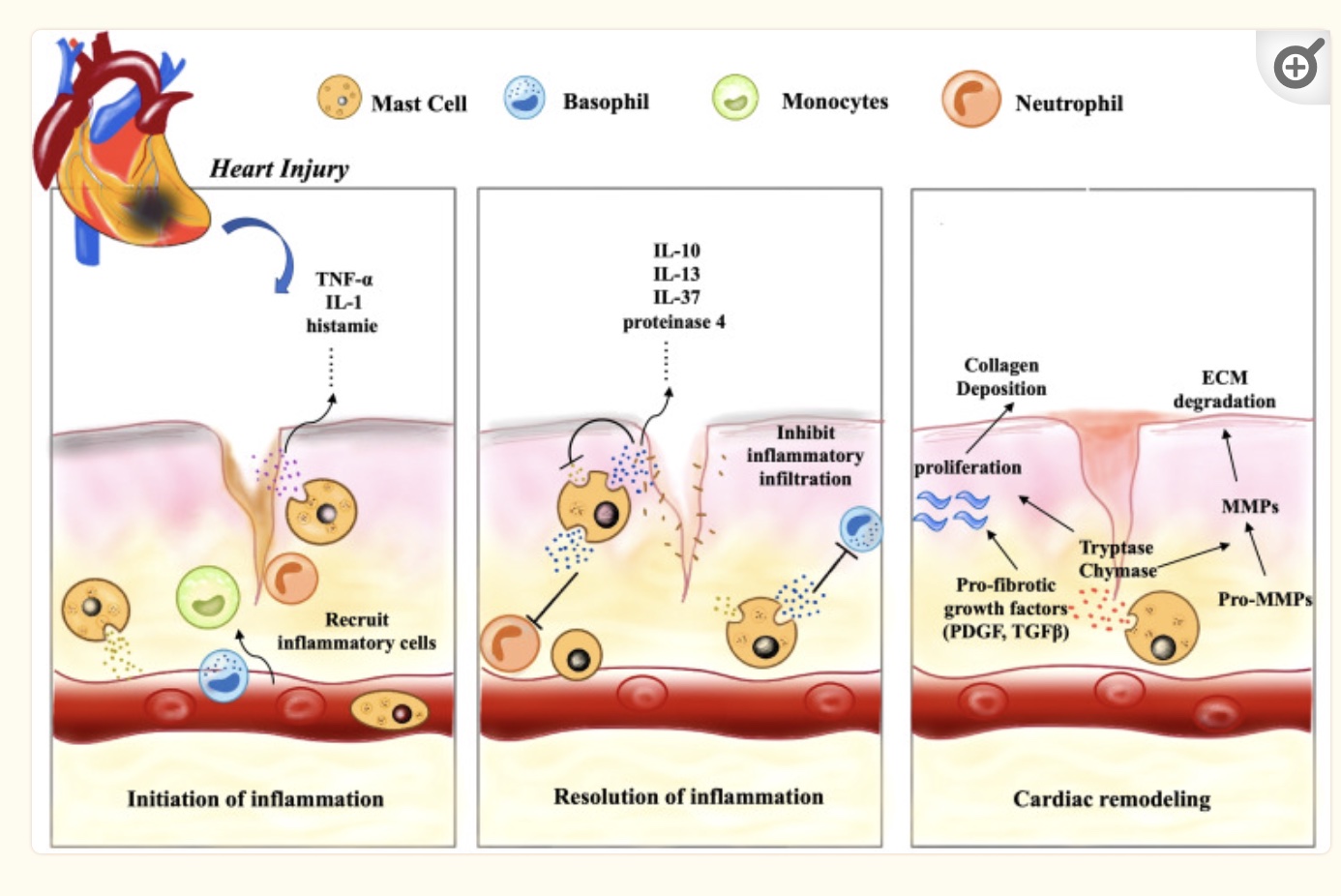
Another player in the inflammatory response in the heart is mast cells. Mast cells are part of the immune response and release histamine, tryptase, and inflammatory cytokines in response to pathogens.
Animal studies show that increased cardiac mast cells cause an increased risk of atrial fibrillation. Additionally, inflammation can prompt mast cells to release more inflammatory cytokines, which results eventually in fibrosis when inflammation isn’t resolved. The tryptase and chymase from mast cells are pro-fibrotic, leading to the accumulation of extracellular matrix in the tissue. However, mast cells can also signal for VEGF-A, which increases capillaries in damaged tissue and promotes repair.[ref]
Digging deeper into genetic susceptibility:
Before we get into the specific genes, let’s talk about how the heart’s nerve cells work and how the genes that code for these mechanisms interact with your heartbeat.
Genetic studies over the past decade have provided much insight into the causes of AFib. A recent paper divides these genetic causes into three categories:[ref]
- Ion channels and transporter genes: changes in how the heartbeat happens including the action potential duration, repolarization, and calcium ion movement
- Transcription factors: changes to the left/right patterning when the heart was formed, ongoing changes in mRNA expression
- Heart tissue structure genes: changes to cell-to-cell communication or myocardial structure
Ion channels:
Ion channels are proteins embedded in the cell membrane of all cells that move ions, such as sodium, potassium, or calcium, in and out of cells. In nerve cells, ions are an essential part of how the electrical impulse is formed and how the nerve fires. Ion movement is essential for cellular function throughout the body, especially in the heart.
Calcium ion channels are essential for driving the contraction and relaxation of the heart muscle, and calcium channels are also important in the generation of nerve impulses. [ref]
In addition, calcium ions in the cell can increase the production of electron donors used to make ATP. Heart muscle cells need a lot of ATP for energy, so good mitochondrial function is essential. However, it is a double-edged sword because mitochondria also produce ROS, which can lead to oxidative stress. The influx of calcium ions and the mitochondrial ROS response likely play a role in the initiation of AFib.[ref]
Structural changes:
Changes in the structure of the heart muscle are also a risk factor for developing AFib.
The muscle that makes up the heart, called the myocardium, is unique and different from skeletal muscle. The cells that make up the heart muscle are called cardiomyocytes. The rhythm of the heart’s contractions is set by pacemaker cells, which are a specialized type of cardiomyocyte in the sinus node.
Heart muscle cells are not replaced often and don’t regenerate well. Less than 1% of the cells turn over annually. Less than 1% of the cells are replaced each year. The heart grows by increasing the size of the cardiomyocytes. This occurs naturally as a person grows from childhood to adulthood, and the cardiomyocytes can increase in size even more with extensive exercise, heart disease, or heart injury. Excess growth in the cardiomyocytes can change the structure of the heart tissue.
How do genes affect the risk of developing atrial fibrillation?
Environmental and lifestyle factors, especially smoking or exposure to toxins, are important in the development of atrial fibrillation. However, genetic susceptibility explains why one person will end up with AFib while another doesn’t.
The PITX2 gene on chromosome 4 was one of the first genes identified to increase the risk of atrial fibrillation and has been studied extensively. The PITX2 gene is a transcription factor that regulates (turns on and off) genes involved in the development of the eyes, teeth, and abdominal organs of a fetus. During development, it is also important for the development of organs and body parts on the left and right sides, including the left-right asymmetry of the heart. Rare mutations in PITX2 cause congenital heart defects such as Tetralogy of Fallot.
While many early studies focused on the role of PITX2 during heart development, PITX2 continues to act as a transcription factor throughout life. It regulates the expression of the IL-6 receptor (tying into inflammation), CAV1, ZFHX3, and other many other genes and miRNAs related to AFib susceptibility and calcium ion handling.[ref]
A recent study looked at what happens to cardiomyocytes (heart muscle cells) when the PITX2 gene is deleted. The researchers found that without the PITX2 gene, the engineered heart tissue beats more slowly than normal. The mutations in PITX2 that cause familial atrial fibrillation are gain-of-function mutations in the PITX2 gene that increase the mRNA levels of the potassium and sodium channels involved in the electrical signal of the heartbeat. Studies in patients with atrial fibrillation show that either higher or lower PITX2 expression could be a cause.[ref]
The CAV1 gene encodes caveolin-1, which is involved in signal transduction in the atrium of the heart. Common genetic variants in CAV1 increase the relative risk of atrial fibrillation by increasing the susceptibility to the signal being altered.
Rare mutations that affect the formation of muscle cells are also linked to AFib. Titin is a protein in muscle cells that provides elasticity. The TTN gene encodes titin, and rare mutations in the TTN gene are linked to changes in the cardiomyocytes and an increased risk of atrial fibrillation. Similarly, rare mutations in the SYNP02L gene, which encodes a cytoskeletal heart protein, also is associated with atrial fibrillation.[ref]
Ion channel mutations, such as in sodium, potassium, or calcium channels in the heart are also linked to AFib.
Atrial Fibrillation Genotype Report
Lifehacks:
Be sure to consider your genetic variants when selecting interventions, and talk with your doctor if you have any questions about atrial fibrillation. The research-backed natural options are listed below, and there are also prescription medications for prevention that your doctor can help you with.[ref]
1) Targeting Inflammation and Promoting Resolution:
Chronic or unresolved inflammation can lead to fibrosis (scarring) in heart tissue, altering the substrate in a way that promotes AFib.
Two ways to target inflammation: increase SPMs and increase antioxidants.
Increase intake of Pro-resolving Mediators (SPMs):
DHA and EPA are omega-3 fatty acids that serve as precursors to specialized pro-resolving mediators (SPMs) such as resolvins, lipoxins, and maresins. These SPMs actively resolve inflammation by turning off inflammatory cytokines and promoting tissue healing. DHA and EPA are abundant in fish and seafood and can also be found in pasture-raised eggs and meat. Our modern diet has shifted to a greatly increased consumption of omega-6 fatty acids and a decreased consumption of DHA and EPA. The lack of pro-resolving mediators likely plays a causal role in AFib from inflammation and fibrosis in the heart.
Related article: Full article on SPMs and the Resolution of Inflammation
- DHA specifically:
Resolvin D1 is a pro-resolving mediator derived from DHA that is key in resolving inflammation in the heart. An animal study showed that supplemental resolvin D1 limited atrial fibrosis and the changes to the heart substrate that cause AFib.[ref] - DHA and EPA from fish: Studies on fish oil supplements for people with a history of atrial fibrillation don’t show much in the way of benefits. This may be because oxidized fish oil in pills doesn’t have a beneficial effect. However, studies show that people who eat more broiled or baked fish have a lower incidence of AFib.[ref]
- Quality matters: Quality is important if you are considering taking a fish oil supplement to increase your DHA and EPA. ConsumerLab.com tests fish oil for heavy metals and oxidation. Their top picks include Carlson Maximum Omega and Kirkland (Costco) fish oil 1,000 mg. DEVA algae-based DHA-EPA also made the top pick list. Failing the freshness test for oxidation were Wiley’s Finest Wild Alaskan Prenatal DHA and Jamieson Omega-3 complete.[ref]
Antioxidant supplements:
Related articles:
Cyp2C19 – check for genetic interactions with Clopidogrel
CYP2C19 Genetic Variant Impact Medications (SSRIs, Blood Thinners) and Toxins
Vitamin K genes influence warfarin dosages.
Vitamin K: Bone Strength, Blood Clots, Calcification, and Genetics