Most people think of platelets forming a clot after they’ve cut themselves — and this is a vital role of platelets. But platelets also do a lot to protect us from getting sick from bacteria and viruses.
In this article, I’ll explain how platelets interact with the immune system – and how a low platelet count (thrombocytopenia) relates to blood clots (thrombosis) in some instances. Then I’ll explain prior research on adenovirus-vector therapy, thrombocytopenia, and platelet reactions.
Recently, governments around the world have paused adenovirus-vector COVID-19 vaccines due to a really rare occurrence of blood clots. Let me be clear upfront: The current stoppage of injecting adenovirus-based vaccinations is due to an undetermined cause of blood clots. The information presented in this article is for background and educational information purposes.
Platelets, immune system, and pathogens:
Platelets are small cell fragments that circulate in your bloodstream and are a component of blood. They actually don’t have a cell nucleus (no DNA), but rather form from the cytoplasm in bone marrow cells. Platelets circulate through the bloodstream and have a ‘lifetime’ of about 7 – 10 days. One of their jobs is to help form blood clots.
Platelets also contribute to our defense against viruses and bacteria, and they are one of the first responders to the invasion.[ref]
For certain bacteria and viruses, your platelets can engulf the particles and sequester them. Macrophages, a part of the innate immune system, then can engulf the platelet.
In other cases, the platelets form aggregates and immune complexes to battle the virus.
After platelet activation, the body will destroy the platelets because they are not reusable. The body gets rid of activated platelets in the spleen and liver.[ref]
Background: Platelets and clotting
When you get a cut, platelets are an integral part of the body’s clotting system. Platelets jump into the break of tissue and release a bunch of different clotting factors, helping to bind to the site of the wound and create a clot to stop the blood flow.
Platelets also activate other coagulation factors. Essentially, they interact with the endothelial cells lining our blood vessels, attaching to cut endothelial cells or to any abnormality on the blood vessel cell wall.
Not all clots form because of a cut or wound. Thrombosis is a medical term for blood clots occurring within a blood vessel, and these can appear due to changes or alterations to blood flow.
Here is an overview showing the injury to the blood vessel (A), the recruitment and activation of platelets (B and C), the aggregation of the platelets (D), and then a plug forming to stop blood loss.[ref]
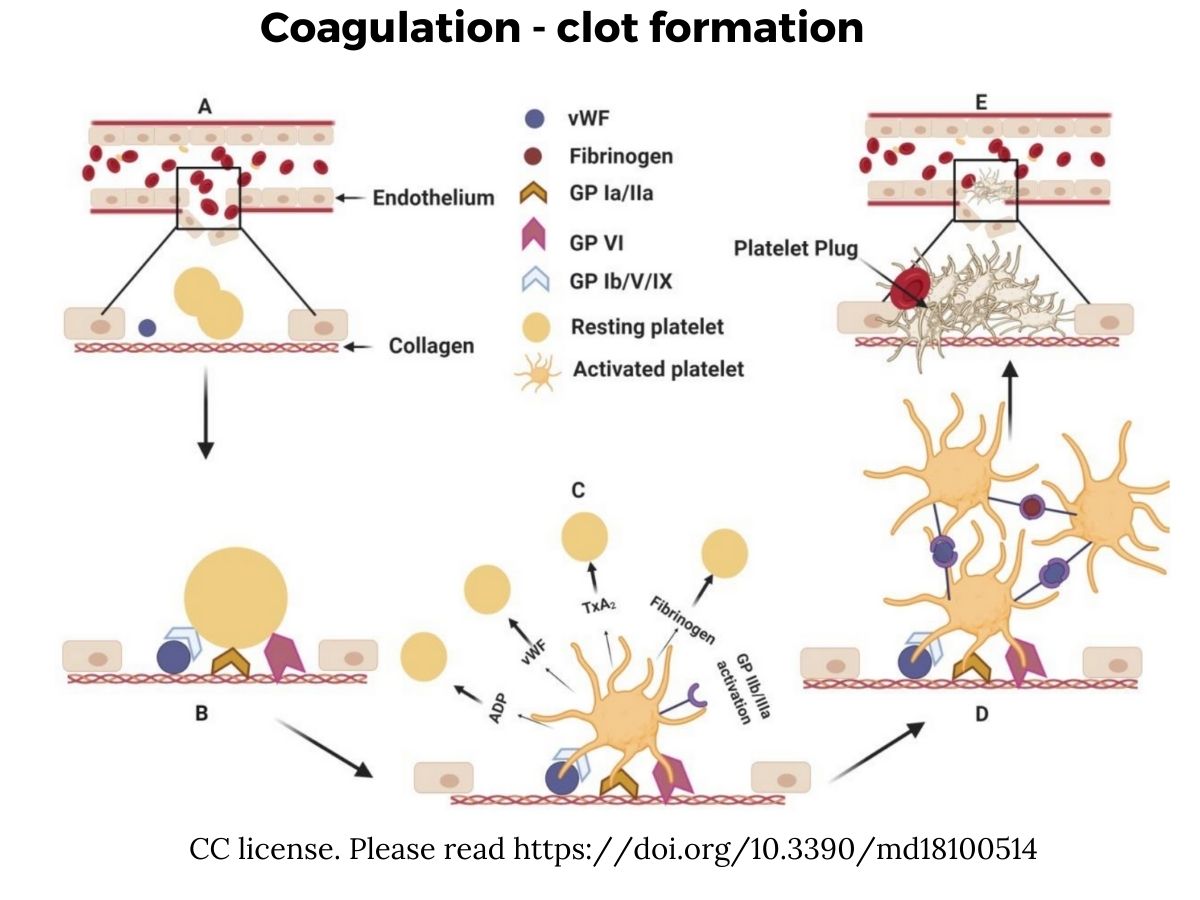
How does a low platelet count tie into blood clots?
Thrombocytopenia is the medical term for a low platelet count. There are many possible causes for thrombocytopenia including genetic mutations, bacterial or viral infection, cancer treatments, sepsis, or excessive bleeding.[ref]
In certain cases, such as vaccine-induced prothrombotic immune thrombocytopenia, blood clots can also occur along with thrombocytopenia.
The low platelet count doesn’t cause blood clots, per se.
The overactivation of the platelets leads to low platelet counts due to the destruction of platelets faster than they can be replaced.
But the activation of platelets also increases the risk for blood clots as the activated platelets stick together and bind to the epithelial cells lining the walls of blood vessels.
In vaccine-induced prothrombotic immune thrombocytopenia, antibodies against the PF4 clotting factor are thought to be involved. This is a rare side-effect that can occur 4 – 21 days after vaccination.[ref]
Blood clots (thrombosis), thrombocytopenia, and adenovirus-vector COVID-19 vaccines
The New England Journal of Medicine published a study in early April 2021 that explains the five cases of “venous thrombosis and thrombocytopenia 7 to 10 days after receiving the first dose of the ChAdOx1 nCoV-19 adenoviral vector vaccine against coronavirus disease 2019 (Covid-19)”.[ref]
The cases reported on in the NEJM paper involved health care workers in Norway who were in their 30s – 50s. They had received the AstraZeneca COVID-19 vaccine. The patients all had severe thrombocytopenia (low platelet count) and blood clots in unusual places. Four of the five patients also had major hemorrhages in the brain.
In March 2021, the European Medicines Agency released a statement indicating the COVID-19 vaccine from AstraZeneca may have associated risks to rare blood clots and thrombocytopenia. The EMA stated that the benefits of the COVID vaccine outweigh the risk. The UK health regulatory authority stated that the AstraZeneca vaccine does not cause blood clots and that the vaccine benefits far outweigh the risk of clots.[ref]
Many countries in the EU have put the AstraZeneca vaccine on hold while the investigation into the risk of blood clots is ongoing.[ref]
In Germany, researchers determined that 4 of the 13 investigated cases of thrombosis and thrombocytopenia could be an autoimmune reaction against platelet antigens. The other nine cases are thought to be due to a cause other than an autoimmune reaction. German health authorities made the decision that the benefits outweigh the negative effects and have resumed AstraZeneca vaccinations.[ref]
In the US, the CDC and FDA in April 2021 issued a joint statement recommending a pause in using the adenovirus-vector vaccine from Johnson & Johnson due to rare cases of thrombosis along with thrombocytopenia in women aged 18-48 which occurred 6 to 13 days after the vaccine.[ref]
Adenoviruses
So, what adenovirus is being used in vaccines?
There are actually a bunch of different adenoviruses — that’s a general family name. Kind of like there are different types of the flu virus and many different herpes viruses.
Several different adenoviruses cause common cold-type symptoms in humans, and one adenovirus even has links to obesity. (Read more about obesity and adenoviruses.) Adenoviruses can also cause gastrointestinal symptoms and pink eye.
Normally, human adenoviruses cause mild disease and are usually only fatal in rare cases such as in immunocompromised people or in AIDs patients.[ref] Adenoviruses can be a cause of ARDS (acute respiratory distress syndrome), though, which is deadly about half the time.[ref] Periodic outbreaks can cause rare complications such as sudden infant death, encephalitis, hemorrhagic cystitis, toxic-shock-like syndrome, and more. From the 60s through the 90s, two vaccines against adenoviruses were used in military personnel to prevent deaths from adenoviruses causing ARDS in military recruits.[ref]
In other animals, adenoviruses can also cause respiratory diseases, such as kennel cough in dogs.
For the most part, the adenoviruses that infect other animals, such as dogs, cats, and monkeys, don’t cause illness in people.
Thus, a bunch of adenoviruses can potentially be used by researchers without making a person sick.
Let me give you an example of how adenoviruses are used in gene transfer…
Adenoviruses have been used as viral vectors to introduce genes into a cell for several decades, and they are one of the more commonly used viruses. These DNA viruses are easily altered to have an additional gene, and then can be injected into cells of a person or animal to transfer the additional gene. While gene therapy is still not routinely used in humans, there have been many trials over the past few decades using adenovirus vectors to deliver a genetic payload for rare diseases or to cure certain cancers.[ref][ref]
The key to using an adenovirus to deliver a genetic payload, whether for curing hemophilia or for delivering a part of the coronavirus, is ensuring the immune system of the host (e.g. you or me) doesn’t attack and kill the adenovirus before it has time to deliver its payload to host cells. About 80% of adults have had exposure to the normal adenoviruses that cause a cold. So researchers and vaccine manufacturers may use adenoviruses that are not normally infecting humans.[ref] Additionally, uncommon human adenoviruses can be altered so that they are not replicated in human cells. This is called a replication-incompetent virus.[ref]
Research studies on gene therapy are still ongoing. Some adenoviral gene transfer trials have not ended well[ref], but others have shown a lot of promise.[ref][ref][ref]
The Johnson and Johnson COVID vaccine uses adenovirus-26 (Ad-26), which is modified to be replication incompetent. This viral vector has been used in previous clinical trials for vaccines against Ebola, HIV, and Zika.[ref]
Ad-26 is a less common human adenovirus that can cause mild intestinal symptoms or pink eye.[ref] The research on Ad-26 symptoms is from the 1960s, when researchers inoculated 400 prisoners in correctional institutions…hmmm.[ref] A study in sub-Saharan Africa in 2007 found that 21% of study participants had antibodies to Ad-26, but the prevalence varies a lot by geographic region.[ref]
The AstraZeneca vaccine chose a different route, using an adenovirus that only infects chimpanzees. Thus, the virus should not cause symptoms in humans.
Adenovirus receptors and cell types that it can enter
For a virus to enter a cell and be transcribed, it needs a way into the cell. For the adenovirus to enter human cells, it can use a receptor called CAR (coxsackie and adenovirus receptor). The CAR protein involves how cells adhere to each other. Both the coxsackie B virus and most types of adenoviruses can take advantage of this receptor to enter cells.[ref]
The CAR protein is essential in the way that the muscle tissue of the heart develops. It is also important in the brain as well as epithelial cells (lining of blood vessels).[ref]
Platelets also express the CAR receptor on their surface.
Another way that adenoviruses can enter cells is through the glycoprotein IIb/IIIa (GPIIb/IIIa). This is also found on platelets. Glycoprotein IIb/IIIa is the receptor for fibrinogen and von Willebrand factor, aiding in platelet activation.[ref]
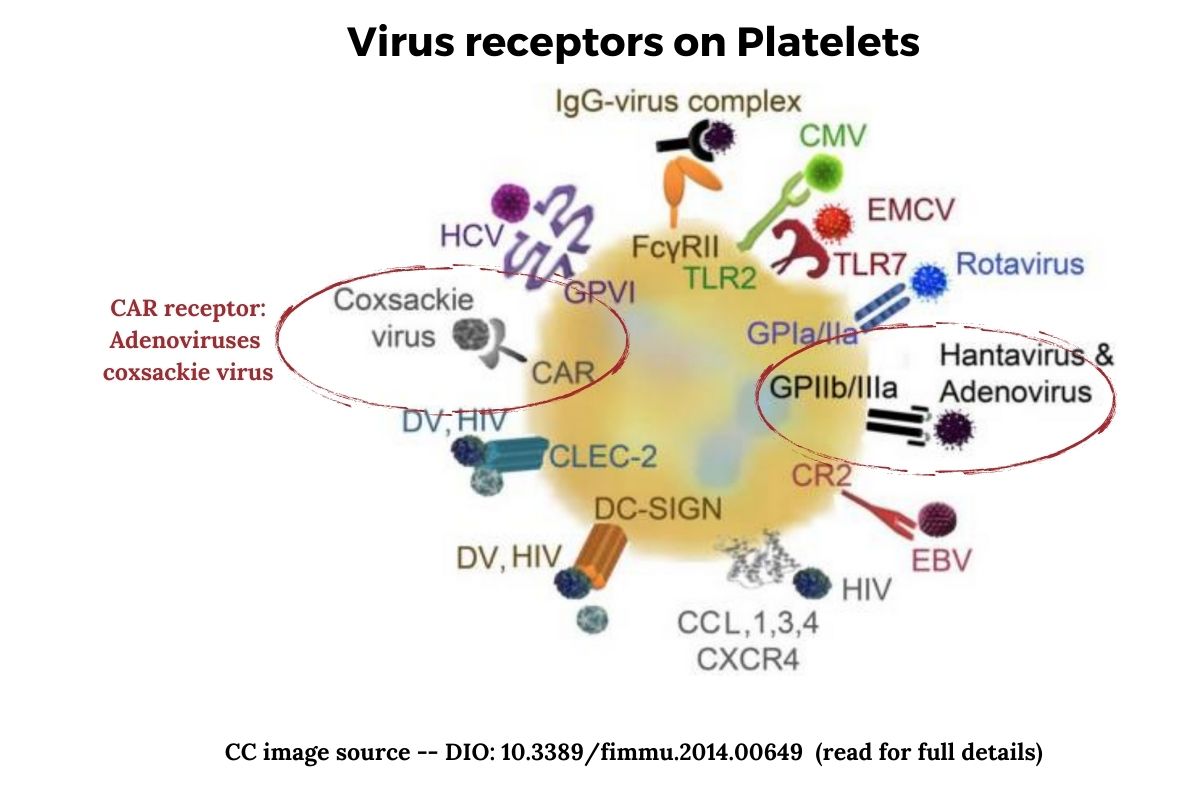
Once an adenovirus infects a cell, the innate immune response kicks into action and releases interferon. Different human adenoviruses will trigger different adaptive immune responses, and the immune system remembers the virus to attack it if encountered again.[ref]
A common effect of viral infections, including adenovirus infections, is a decrease in platelet count, known as mild thrombocytopenia. For most viral infections this doesn’t lead to significant bleeding (except for viral hemorrhagic fevers such as Ebola).[ref]
Adenoviruses and thrombocytopenia:
One problem with using adenoviruses for gene therapy is thrombocytopenia.
Research shows that injected adenoviruses initiate platelet aggregation. Additionally, platelets can internalize the adenoviruses, which also results in more platelet aggregation and then destruction.[ref]
This platelet activation and subsequent thrombocytopenia (low platelets) is well known from early adenovirus research for gene therapy and has been noted in both animal trials and in humans.[ref]
A 2007 research study on adenoviruses for gene therapy states: “Adenovirus-induced thrombocytopenia is a potentially serious complication of gene therapy protocols using this type of vector.”[ref]
Research shows that one cause of platelet activation by adenoviruses is von Willebrand factor.
Von Willebrand factor (VWF) is an adhesive protein released from activated platelets causing platelets to adhere to endothelial cells (lining of blood vessels). When platelets adhere to the endothelial cells, the endothelium also releases ultra-large multimers of VFW.
Additionally, adenoviruses themselves can activate the endothelial cells to release ultra-large multimers of von Willebrand factor.[ref]
So, several things are going on together: adenoviruses activate platelets and cause them to be destroyed. The activated platelets can also release von Willebrand factor, which causes them to adhere to endothelial cells (lining of blood vessels). The endothelial cells also release large multimers of von Willebrand factor.
In mice, over the first 24 hours after an injection of adenovirus, the amount of VWF rises dramatically within a couple of hours and the number of platelets decreases over the 24-hour period. The low point for platelets in mice seems to be about 24 hours before they start to recover.[ref]
Von Willebrand factor is key here. In mice with the VWF gene removed, there was no thrombocytopenia following adenovirus administration.[ref]
Animal studies using primates show that replication-incompetent adenovirus injection using adenovirus 5 reduces platelet count in a way that corresponds to the dosage used. Higher doses of the adenovirus reduced platelet counts significantly (90%). The animals did recover, though, beginning 24 hours after the injection.[ref]
Studies using human plasma similarly show that adenovirus 3 induces platelet activation.[ref]
The clinical trials for an Ebola vaccine that uses an adenovirus vector also show that it decreased platelet count. “Platelet counts were also decreased 24 and 48 hours after the first injection of the test item in both males and females. It was followed by an increase on days 8 and 12. After the second injection, the decrease in platelet count was seen 24 hours later in males only and was less pronounced than after the first injection.” Note that the platelet count decrease was not considered important because the trial participants’ platelet counts recovered within two weeks.[ref] Another study reported that no clinically meaningful cases of thrombocytopenia occurred in the 1509 participants in an adenovirus vaccine trial for Ebola.[ref] A separate report on 300 children in an adenovirus vaccine trial for Ebola found “No clinical symptoms of thrombocytopenia were reported.”[ref]
ADAMTS13, von Willebrand factor, and thrombotic thrombocytopenic purpura:
Endothelial cells can release von Willebrand factor in ultra-large particles, and these particles then need to break apart so that they don’t cause problems.
ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif member 13) is responsible for cleaving ultra-large VWF.[ref]
Thrombotic thrombocytopenic purpura (TTP) is a blood disorder that causes blood clots to form in small blood vessels. It is caused by a significant decrease or absence of ADAMTS13. This is a serious condition with a mortality rate of 90% without treatment.
TTP can be caused by genetic mutations or autoantibodies (autoimmune).
When ADAMS13 is deficient, “large Von Willebrand Factor (VWF) multimers accumulate leading to platelet aggregation, hemolysis, and microthrombi formation. The microthrombi cause ischemia is leading to damage to end organs, with the most common being the central nervous system (CNS) and kidneys. Thrombocytopenia results from platelet consumption during thrombus formation. Anemia results from hemolytic destruction of red blood cells as they pass through small vessels that are partially occluded by thrombi.”
What about genetics? Are some people more prone to thrombocytopenia?
This is normally where I would include genetic variants that apply to the topic. Instead, I’ve published an article on genetic variants in the ADAMTS13 and VWF genes as a separate piece. I’m doing this to separate the vaccination topic from genetics. There is no research directly linking genetics to adenovirus-vector vaccine responses, and I don’t want to imply that the link is known.
Why would women be more prone to blood clots?
Reactions to the AstraZeneca vaccine predominately affect younger women.
A study investigated the platelet aggregation response in women and men to see if there were differences due to sex, age, menstrual cycle, or oral contraceptive use. The researchers found that premenopausal women had more platelet aggregation compared to men of any age or older women.
The study results showed “Women of younger age (<45 years) had significantly higher agonist-induced aggregation response than both men and post-menopausal women (60-65 years). The agonist-induced aggregation response did not differ between phases of the menstrual cycle or OC [oral contraceptive] use.”[ref]
Other studies also show that premenopausal women have higher platelet aggregation than women who have gone through menopause.[ref] Researchers theorize the increased platelet aggregation capacity could be to “prevent excessive bleeding during menstruation”.[ref]
Exercise and platelet function: Researchers have also looked at the effects of exercise in women on platelet function. Strenuous exercise significantly increases platelet adhesiveness in premenopausal women, but moderate exercise does not.[ref]
Conclusion:
The link between viruses and blood clots has long been known, and there are multiple mechanisms at play.
Research over the past few decades has found that adenoviruses are useful for gene transfer, and ongoing trials show promise for using adenovirus vectors in curing rare genetic diseases.
One known effect of adenovirus vectors is that they cause a decrease in platelets, which seems to be dose-dependent. For the participants in the clinical trials, platelet levels recovered within a week or two.
The exact cause of the rare blood clots with the adenovirus-based vaccines is still undetermined.
Related Articles and Genes:
9 Genes that Impact the Response to Vaccines
Genetics plays a huge role in how an individual responds to a vaccine. Learn more about vaccines, immunity, and genetic variants that affect the response.
TNF-alpha: Inflammation and Your Genes
Do you feel like you are always dealing with inflammation? Joint pain, food sensitivity, etc? Perhaps you are genetically geared towards a higher inflammatory response. Tumor necrosis factor (TNF) is an inflammatory cytokine that acts as a signaling molecule in our immune system.