Key takeaways:
~ There are multiple physiological pathways that can cause what is commonly called “brain fog”.
~ By understanding the different possible causes, you may be able to determine your root cause.
~ Genetic variants can help you narrow down which causes are more likely for you.
~ Solutions based on the different root cause pathways are explained in detail in the lifehacks section.
This is a long article, bookmark it now.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
What is brain fog?
Brain fog is a term that is hard to define — especially if you are currently dealing with it! People describe it as having trouble remembering words or names, having difficulty with multitasking, being forgetful, being inattentive or uninterested in things, or just having plainly hazy thinking. If you are dealing with brain fog, understanding the physiological causes can lead you to solutions that actually work for you.
Brain fog doesn’t seem to have a recognized scientific definition. Doctors can describe it as mild cognitive impairment, mental fatigue, or neurocognitive dysfunction.[ref]. Researchers describe brain fog as: “cognitive dysfunctions such as memory loss, speech deficit (lack of words, problems with fluency), and a decline in performance and learning abilities.“[ref]
The neurocognitive symptoms involved in brain fog can impact your ability to work or complete normal daily activities. Brain fog impedes relationships, impairs decision-making, causes sensory overstimulation, and decreases the ability to communicate well.[ref]
Research shows that cognitive impairments can have multiple biochemical causes.
Takeaway:
Brain fog is real, and it has several different physiological causes. It impacts your quality of life. Figuring out your underlying root cause may give you the key to reversing brain fog.
Let’s get started…
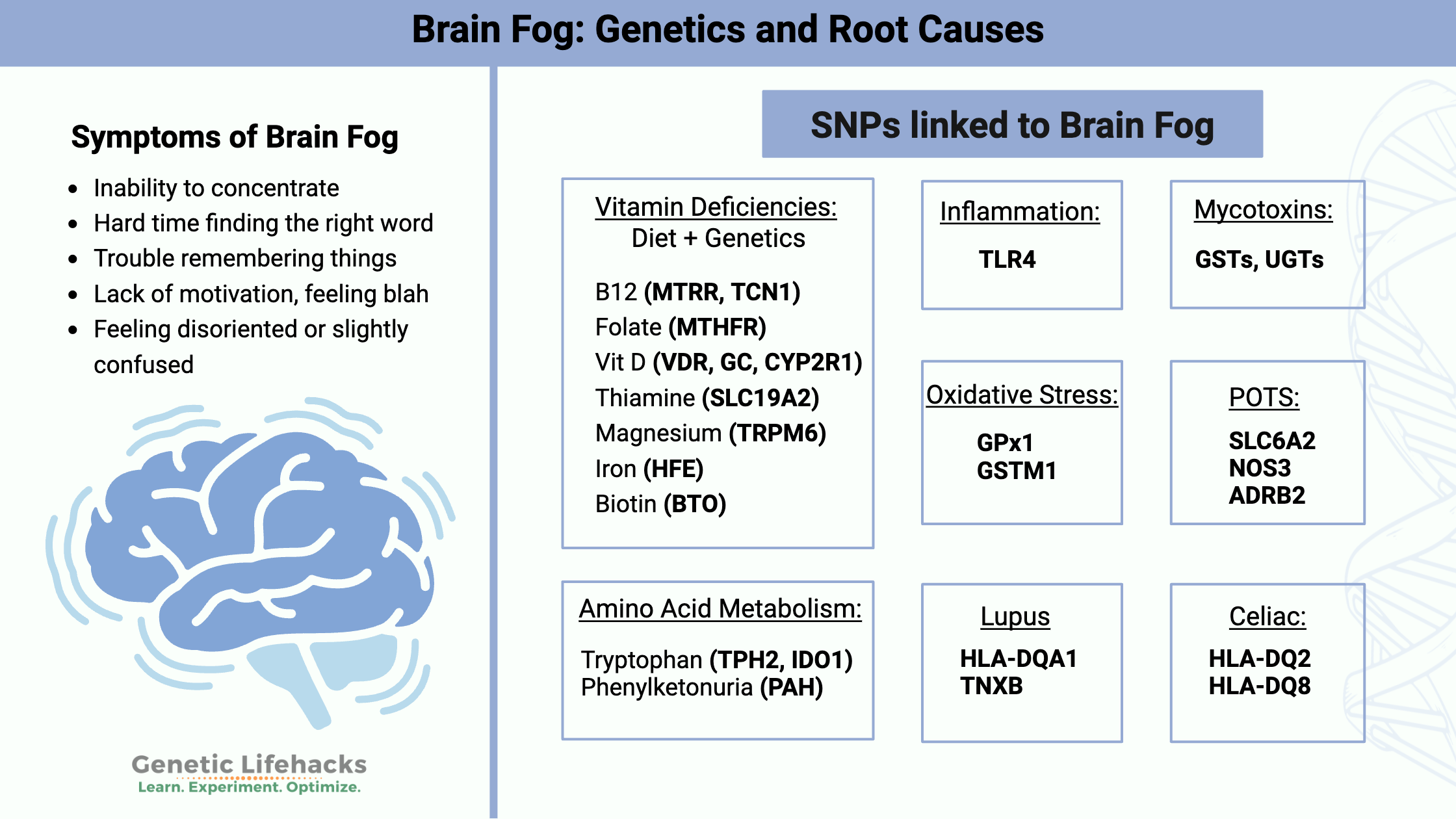
Brain Fog Symptoms
Symptoms of brain fog can vary from person to person. Here are some of common complaints:
- Inability to concentrate
- Hard time finding the right word
- Trouble remembering things
- Lack of motivation, feeling blah
- Feeling disoriented or slightly confused
Now that we have defined what we are talking about, let’s look at the science behind what is happening in the brain.
What causes brain fog? Physiological changes in the brain
When you read most articles about brain fog, they will tell you that brain fog is due to a lack of sleep or hormone changes.
I’m going to assume that you know that you should sleep well. If you are pregnant or going through hormone changes, well, I assume you also know that. Instead, I’m going to focus here on the physiological reasons that your brain may not be working optimally.
9 Underlying causes of brain fog include:
- vitamin or nutrient deficiency
- hypoxia (lack of oxygen)
- elevated inflammatory cytokines
- viral or bacterial infection (e.g., Long Covid)
- amino acid deficiency
- metabolic dysfunction in the brain
- hypothyroidism
- medication-induced brain fog
- sleep/circadian rhythm problems
I’ll explain each of these in more detail. Then I’ll give you the brain fog genotype report – tied back to these underlying causes.
Keep in mind that there may be several physiological factors contributing to your brain fog.
Stick with me here… this gets a bit long.
1) Vitamin deficiencies linked to brain fog
Several different vitamin deficiencies can result in the symptoms associated with brain fog.
Genetic variants can cause increased susceptibility to specific vitamin deficiencies. (See details in the Brain Fog Genotype Report below.) Diet and lifestyle factors also can cause vitamin deficiencies.
Vitamin B12 Deficiency:
Vitamin B12, also called cobalamin, is a water-soluble vitamin found in animal-based foods (meat, dairy, eggs). B12 is used as a cofactor in cellular processes related to DNA synthesis, fatty acid metabolism, folate/methylation cycle purposes, and protection of the myelin sheath in neurons.[ref]
Vitamin B12 deficiency symptoms include neurological symptoms such as memory loss, disorientation, dementia-like symptoms, and numbness or tingling of the hands and feet. Additionally, tongue soreness, appetite loss, and constipation could be symptoms of B12 deficiency. Eventually, long-term B12 deficiency can cause anemia, which decreases the oxygen-carrying capacity in the blood.
Folate deficiency:
Vitamin B9, or folate, is another possible cause of brain fog. Folate deficiency can lead to megaloblastic anemia, which decreases oxygen-carrying capacity. It can cause fatigue, weakness, and shortness of breath. Low folate can also cause hyperhomocysteinemia. Additional symptoms that can happen in folate deficiency include depression and cognitive dysfunction.[ref][ref]
Vitamin D deficiency:
Low vitamin D is also linked to brain fog. Many studies show that low vitamin D likely contributes to mild cognitive impairment and brain fog. For example, a study of lupus patients found that vitamin D deficiency predicted cognitive dysfunction.[ref][ref]
Thiamine deficiency:
This water-soluble vitamin is essential in energy metabolism, and a lack of thiamine can impact cellular energy production. In the brain, thiamin deficiency causes increased oxidative stress as well as a buildup of lactic acid due to problems with cellular energy. Thiamine deficiency can result in cognitive and neurological problems, along with cardiovascular issues when long term.[ref]
Magnesium insufficiency:
As a cofactor involved in hundreds of cellular reactions, a magnesium deficiency is associated with cognitive dysfunction in older adults.[ref]
Excess iron:
Your body and brain need just the right amount of iron. While uncommon, building up excess iron due to mutations related to hemochromatosis can also cause cognitive impairment.[ref]
How can you apply this information?
- Take a look at your genetic variants below in the Brain Fog Genotype Report section to see if you have variants that increase susceptibility to vitamin deficiencies. Also, consider your diet. For example, vegan diets don’t contain vitamin B12, and junk food diets may be low in magnesium.
- Try using an app like Cronometer.com to track what you eat for several days. Cronometer.com breaks down your nutrient intake and shows you if you are lacking in a vitamin or mineral.
- Going overboard with high doses of vitamins isn’t necessarily the answer (and may cause problems).[ref] Instead, targeting a deficiency and raising your levels to normal over time may be a better solution.
Moving on to the topic of getting oxygen to the brain…
2) Oxygen, brain blood flow, and POTS
In addition to nutrition, your brain needs a lot of oxygen to work properly. Reduced brain oxygen levels, also known as hypoxia, could be caused by a number of different factors.
I will cover several possible causes of hypoxia, but there could be other reasons for decreased blood flow to the brain.
POTS (Postural Orthostatic Tachycardia Syndrome) and ME/CFS
Do you get light-headed when you stand up? POTS is defined by a significant change in heart rate when standing up or sitting up from lying down, which can cause symptoms such as orthostatic intolerance, tachycardia, palpitations, dizziness, and cognitive impairment.[ref].
POTS can be a stand-alone diagnosis, or it can go along with Ehlers-Danlos syndromes (collagen synthesis disorder), Mast Cell Activation Syndrome, or ME/CFS (chronic fatigue syndrome).
For people with POTS, the change in blood flow upon sitting or standing may cause a decrease in the blood flow to the brain. Additionally, POTS can cause altered blood flow even when not changing position. POTS patients often report problems with attention, concentration, memory, mental clouding, and information processing — all of which fall under the umbrella of ‘brain fog’. [ref]
Brain imaging studies show why people with POTS or ME/CFS have brain fog.
In the 1990s, researchers found that 80% of ME/CFS patients had reduced cerebral blood flow. Subsequent studies showed that POTS and ME/CFS patients lack the increased blood flow to the brain seen in a control group when faced with mental stress tests. In other words, thinking usually takes more blood flow, and people with chronic fatigue and POTS don’t have that normal response.[ref]
Microclots, blocking oxygen:
Another possible cause of hypoxia is microclots, or small blood clots. Clots in small blood vessels can reduce blood flow in the brain.
There are several possible causes of small blood clots:
- amyloid fibrinogen clots that are not dissolved normally
- microclots not being broken down appropriately by alpha-2 antiplasmin[ref]
- decreased blood flow in the elderly
Let me explain both of these further:
Amyloid fibrinogen:
Proteins in the body are folded into specific formations that are important in how they interact. Amyloid is a term applied to misfolded proteins — misfolded in a way that resists being broken down or refolded into the right formation.
Fibrinogen is a protein that is essential in forming a blood clot. It circulates all the time in the bloodstream, ready for when a clot needs to form. When activated by thrombin, fibrinogen turns into fibrin, which can cross-link and form the fibrous mesh needed in blood clots.
Research from 2016 shows that fibrinogen can form an amyloid protein structure when exposed to certain substances, including lipopolysaccharides found on some bacterial pathogens.[ref]
Recently, researchers have looked at the formation of microclots in relation to COVID-19 and the spike protein.
- One study (pre-print) found that the spike protein causes abnormal, amyloidogenic clotting in normal blood. This clotting initiated the formation of antibodies towards fibrinogen, which persisted “long after the acute infection”.[ref]
- Another research paper explains: “Although the symptoms of Long COVID are multifarious, we here argue that the ability of these fibrin amyloid microclots (fibrinaloids) to block up capillaries, and thus to limit the passage of red blood cells and hence O2 exchange, can actually underpin the majority of these symptoms.” The symptoms included here are fatigue, brain fog, and muscle pain.[ref]
Alpha-2 antiplasmin:
Clots are continually built up and broken down in processes involving multiple proteins and enzymes. Plasmin is part of the degradation process for clots, and alpha-2 antiplasmin inactivates plasmin. Thus, an excess of alpha-2 antiplasmin could slow the breakdown of clots.
A recent study found that people with Long Covid who have microclots also have an excess of alpha-2 antiplasmin. The researchers used plasma samples from Long Covid patients and found that their microclots were resistant to fibrinolysis. Further testing showed that alpha-2 antiplasmin was elevated in Long Covid patients compared to a healthy control group.[ref]
Decreased blood flow in the brain:
Cerebral blood flow also decreases in aging. It is thought to be only part of the problem regarding a cognitive decline in the elderly. Research also shows that cerebral metabolism is important.[ref]
Sleep apnea, hypoxia, and brain fog:
People with sleep apnea periodically stop breathing while sleeping. This interruption causes intermittent hypoxia or a lack of oxygen. The intermittent hypoxia shifts the hippocampus (a region of the brain) into a pro-oxidant state. This increase in oxidative stress increases HIF1A, a protein important in controlling how genes are turned on in response to low oxygen. Inhibiting HIF1a reduced cognitive issues due to intermittent hypoxia (animal study).[ref] Long-term sleep apnea decreases the brain’s ability to create new neurons.[ref]
Related article: HIF1a and hypoxia genes
3) Inflammation negatively affects cognition
When you are sick, you may notice that you have ‘brain fog’ symptoms. Along with feeling tired and sick, you cannot think clearly.
This inability to think clearly is an innate response to elevated inflammatory cytokines, with your body preferentially shuttling resources toward fighting off a pathogen.
Researchers talk about ‘sickness behavior’, involves loss of appetite, fatigue, muscle weakness, and decreases in cognition. Essentially, your body is hard-wired to react to inflammatory cytokines in this way to optimize the ability to fight off viral or bacterial pathogens.[ref]
However, inflammation can occur at low levels due to oxidative stress, sleep deprivation, exposure to toxins, mental stress, and injury. For example, increases in inflammatory cytokines, such as TNF-alpha, may cause sickness behavior when elevated due to reasons other than responding to a pathogen.
Here are a few studies showing how elevated inflammation causes changes in cognition.
- Higher levels of TNF-alpha and CRP correlate with worse cognition in a study of middle-aged adults in the US.[ref]
- Elevated cytokine levels after surgery cause cognitive dysfunction in patients.[ref]
- In animal models of neurodegeneration, injections with TNF-alpha caused acute impairment of working memory. Interestingly, the TNF increase did not cause memory impairment in young, healthy mice.[ref]
Related article: Chronic inflammation and genetics
4) Viral infections (e.g. COVID Brain Fog)
Persistent viral infections, such as Epstein-Barr virus or HIV, often go hand-in-hand with brain fog. Lyme disease and SARS-CoV-1 can also cause long-term brain fog.[ref]. Of people with HIV, ~ 40% experience HIV-associated neurocognitive disorder.[ref]
Up to 85% of patients with ME/CFS (chronic fatigue syndrome) report brain fog. Memory tests show that people with ME/CFS have decreased processing speed, impaired information processing, and memory problems. In approximately 70% of patients, there is a known infection, such as Epstein Barr, that initiates the onset of ME/CFS.[ref]
The SARS-CoV-2 virus is also tied to cognitive dysfunction. The most significant increase in people with brain fog recently has been in people with COVID or exposure to the spike protein.
Let’s dig into this further….
Long Covid, Spike Protein, and Brain Fog:
Persistent symptoms after recovering from a COVID-19 infection are referred to as “Long Covid” or post-acute sequelae of COVID-19 (PASC). The two main complaints of Long Covid for the majority of people are fatigue and ‘cognitive complaints’ or brain fog.[ref]
There are three reasons being researched for Long Covid brain fog. These may also apply to other persistent viral infections and exposure to the spike protein.
- Neuroinflammation
- Hypoxia
- Microclots
Neuroinflammation:
One theory of Long Covid brain fog is that it is caused by a prolonged, excessive immune response. Higher than normal levels of inflammatory cytokines could be the link between Long Covid and brain fog.[ref] If the spike protein crosses the blood-brain barrier, animal studies show that the spike protein can directly cause neuroinflammation.[ref] (There isn’t a lot of evidence that the spike protein is crossing the BBB in most causes of Covid-19)
The SARS-CoV-2 spike protein S1 subunit has been shown to activate the immune response. Specifically, animal and cell studies show that the spike protein S1 subunit increases neuroinflammation by activating the general immune system response to viral patterns (PAMP). The study showed that inflammatory cytokines (TNF, IL-1B, INF-gamma, NLRP3, IL-2) were elevated in different brain regions after exposure to the spike protein.[ref]
Hypoxia:
Another theory is that Long Covid brain fog is linked to vascular insufficiency — not enough blood and oxygen getting to the brain. A small MRI-based study found that patients with Long Covid brain fog showed reduced lactate and higher glutamine + glutamate (GLX) levels in the brain.[ref]
Microclots:
As mentioned above, are another possible cause of brain fog after SARS-CoV-2 spike protein exposure. Research shows that the S1 subunit of the spike protein causes coagulation. The study found that “when spike protein S1 is added to healthy PPP [platelet rich plasma], it results in structural changes to β and γ fibrin(ogen), complement 3, and prothrombin.”[ref] Microclots may be decreasing oxygen and nutrients to regions of the brain, but coagulation issues could also be chronically activating the immune response.
5) Mycotoxin (mold) exposure and brain fog:
Mycotoxins are microscopic mold metabolites that are harmful to your body and brain. They are naturally occurring toxins produced by filamentous fungi (molds). They are classified as toxins because even at very low doses, they can cause ill effects or even death in humans and other animals.[ref]
One effect of mycotoxins is mitochondrial dysfunction. Practitioners link mycotoxin exposure to brain fog in some patients. If you think mold could be a problem for you, read through the article on Mold genes – mycotoxin interactions.
6) Histamine, MCAS, and brain fog
Mast cells are a type of immune cell that are activated in allergic reactions as well as in the immune response. Brain-resident mast cells are implicated in neuroinflammation in some instances. Once activated, mast cells release inflammatory mediators, including histamine, serotonin, and TNF-alpha.
Mast cell activation syndrome (MCAS) is characterized by different symptoms, depending on which tissue has mast cells activated. MCAS issues range from hives, anaphylaxis, and gut issues to autonomic nervous system problems and cognitive dysfunction.[ref].
One link to cognitive alterations with MCAS is that mast cells release histamine (along with other inflammatory molecules). In the brain, histamine acts as a neurotransmitter. Histaminergic neurons are located in several regions of the brain, including the hypothalamus and cerebral cortex. Histamine in the brain regulates wakefulness, appetite, stress response, and memory. Cognitive impairment can be caused by altered histamine levels in the brain.[ref]
Excessive mast cell activation can be caused by activated viruses, IgG, immune complement factors, microbial components, drugs, hormones, physical stimuli, emotional stress, and elevated cytokines.[ref]
Related article: Histamine intolerance genes
7) Autoimmune diseases that cause brain fog:
The immune system’s mistaken identification of your own cells as foreign intruders leads to healthy cells being attacked in autoimmune diseases. Here are a couple of autoimmune diseases that are strongly linked to brain fog:
Celiac disease:
The majority of celiac patients report brain fog as a symptom after gluten ingestion. Celiac disease is an autoimmune reaction to gluten (found in wheat) that results in the destruction of cilia in the intestines. Brain fog in celiac disease is backed up by cognitive tests showing a very mild cognitive impairment, similar to having a blood-alcohol level of 0.5%. The authors of the study note that cognitive impairment in celiac patients is similar to that seen in other autoimmune diseases (MS, lupus) and due to immune system activation.[ref]
Related article: Genes and Celiac disease
Lupus:
Up to 88% of lupus patients report brain fog or cognitive dysfunction. Research points to blood-brain barrier integrity problems in lupus, allowing autoantibodies and inflammatory cytokines to increase in the brain.[ref]
Related article: Lupus
Hypothyroidism as a cause of brain fog:
The majority of people with hypothyroidism, including Hashimoto’s, report brain fog as a symptom before treatment. One study of over 5,000 hypothyroid patients found that almost 80% had experienced brain fog. Thyroid hormone replacement treatment did not help all hypothyroid patients, and for some, T4 treatment seems to increase brain fog.[ref]
Related article: Learn how your genes impact converting T4 to T3
8) Amino acid metabolism possibly causing brain fog:
Phenylalanine is an essential amino acid found in many foods containing protein. The PAH system in the liver metabolizes phenylalanine into tyrosine, the precursor for the brain to make the neurotransmitter dopamine.
People with phenylketonuria (PKU), a hereditary condition caused by mutations in the PAH gene, have a buildup of phenylalanine in the brain. The PAH gene encodes the phenylalanine hydroxylase enzyme, which is key to converting phenylalanine into tyrosine.
Two copies of PAH mutations are necessary to cause phenylketonuria. But people who are carriers of one PAH mutation have around 50% of normal phenylalanine hydrolase function.
People who carry one copy of a PAH mutation have a lower verbal recall, on average, when compared to a healthy control group. Additionally, they performed worse on divided attention and sensitivity to processing speed.[ref]
One key may be that diets high in phenylalanine are more likely to cause cognitive issues in people with one PAH mutation. For example, aspartame is converted to phenylalanine, so PAH mutation carriers may find that diet soda is a problem.
Some of the known PAH mutations are listed below in the genetic section.
Related article: Phenylketonuria
Tryptophan is another essential amino acid that may impact brain function. Tryptophan can be converted either into serotonin or kynurenine. When tryptophan becomes kynurenine, some of it can end up as quinolinic acid, which is neurotoxic. If things become unbalanced and too much quinolinic acid is produced, neuroinflammation is possible. The endpoint of the kynurenine to the quinolinic acid pathway is to create NAD+ for use in cellular energy creation.
Tryptophan metabolism also ties into the spike protein and Long Covid. A study (in pre-print) of 128 people assessed them at 2, 4, and 12 months after Covid. The results showed mild to moderate cognitive impairment in up to a quarter of the participants. The researchers found that kynurenine pathway metabolites, including quinolinic acid, were elevated in participants with cognitive impairment. The conclusion was that the association suggested: “a potential causal link thereby indicating it as a biomarker and therapeutic target”.[ref]
Cells will shunt tryptophan towards the kynurenine pathway when NAD+ levels are low. One way to prevent this is to get enough niacin, either through your diet or supplements.
Related article: Tryptophan, kynurenine, and serotonin
Brain Fog Genotype Report:
Lifehacks: How to get rid of brain fog
You’ve made it to the application portion of this article! Here I will explain the research on possible solutions (lifestyle, diet, supplements) to the genetic susceptibility variants above.
General brain fog research:
Conclusion:
Brain fog can be a symptom that underlies nutrient deficiency, amino acid metabolism issues, coagulation problems, or viral or bacterial illness. Don’t dismiss brain fog as just being mild, a symptom of getting older, or something you can live with. It is your brain saying something isn’t quite right, and you need to find and fix the underlying problem.
Talk with your health care provider if you need help getting lab tests run or need advice on supplements (especially if you are on prescription medications). If your current doctor isn’t open to discussing the underlying cause of your brain fog, I encourage you to reach out to another health practitioner so that you can find someone to partner with you in your health.
Related Articles and Topics:
Small Fiber Neuropathy: Genetics, Causes, and Possible Solutions
Small Fiber Neuropathy (SFN) results in burning pain, numbness, odd sensations, or autonomic nervous system issues. Learn more about the possible causes and potential solutions to this debilitating disorder.
TTR gene: hereditary transthyretin amyloidosis
New research shows that hereditary transthyretin amyloidosis (hATTR) may be more common, especially in people of African ancestry. Understand your genetic risk before irreversible damage.
Examining the Research on Vitamin D and SARS-CoV-2
Is vitamin D helpful for SARS-CoV-2? Investigate and discover more with a serious look at the research studies on vitamin D and SARS-CoV-2 (and genetics, of course).
Do you need more Vitamin C? Nutrigenomic reasons
Like most nutrients, our genes affect how vitamin C is absorbed, transported, and used by the body. It can influence your risk for certain diseases and make a difference in the minimum amount of vitamin C you need to consume each day.
References:
Akin, Cem, et al. “Mast Cell Activation Syndrome: Proposed Diagnostic Criteria.” The Journal of Allergy and Clinical Immunology, vol. 126, no. 6, Dec. 2010, pp. 1099-104.e4. PubMed Central, https://doi.org/10.1016/j.jaci.2010.08.035.
Al‐Amin, Mamun, et al. “Vitamin D Deficiency Is Associated with Reduced Hippocampal Volume and Disrupted Structural Connectivity in Patients with Mild Cognitive Impairment.” Human Brain Mapping, vol. 40, no. 2, Sept. 2018, pp. 394–406. PubMed Central, https://doi.org/10.1002/hbm.24380.
Ankar, Alex, and Anil Kumar. “Vitamin B12 Deficiency.” StatPearls, StatPearls Publishing, 2022. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK441923/.
Arias-Cavieres, Alejandra, et al. “A HIF1a-Dependent Pro-Oxidant State Disrupts Synaptic Plasticity and Impairs Spatial Memory in Response to Intermittent Hypoxia.” ENeuro, vol. 7, no. 3, June 2020, p. ENEURO.0024-20.2020. PubMed Central, https://doi.org/10.1523/ENEURO.0024-20.2020.
Bennett, J. W., and M. Klich. “Mycotoxins.” Clinical Microbiology Reviews, vol. 16, no. 3, July 2003, pp. 497–516. DOI.org (Crossref), https://doi.org/10.1128/CMR.16.3.497-516.2003.
Blomberg, Jonas, et al. “Antibodies to Human Herpesviruses in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients.” Frontiers in Immunology, vol. 10, Aug. 2019, p. 1946. PubMed Central, https://doi.org/10.3389/fimmu.2019.01946.
Brown, Shona, and Lorna A. Torrens. “Ironing out the Rough Spots – Cognitive Impairment in Haemochromatosis.” BMJ Case Reports, vol. 2012, July 2012, p. bcr0320126147. PubMed Central, https://doi.org/10.1136/bcr.03.2012.6147.
Callan, Caitriona, et al. “‘I Can’t Cope with Multiple Inputs’: A Qualitative Study of the Lived Experience of ‘Brain Fog’ after COVID-19.” BMJ Open, vol. 12, no. 2, Feb. 2022, p. e056366. PubMed Central, https://doi.org/10.1136/bmjopen-2021-056366.
Cysique, Lucette A., et al. Post-Acute COVID-19 Cognitive Impairment and Decline Uniquely Associate with Kynurenine Pathway Activation: A Longitudinal Observational Study. medRxiv, 7 June 2022, p. 2022.06.07.22276020. medRxiv, https://www.medrxiv.org/content/10.1101/2022.06.07.22276020v1.
Ettleson, Matthew D., et al. “Brain Fog in Hypothyroidism: Understanding the Patient’s Perspective.” Endocrine Practice : Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, vol. 28, no. 3, Mar. 2022, pp. 257–64. PubMed Central, https://doi.org/10.1016/j.eprac.2021.12.003.
Fontes-Dantas, Fabricia, et al. SARS-CoV-2 Spike Protein Induces TLR-4-Mediated Long-Term Cognitive Dysfunction Recapitulating Post-COVID Syndrom. 2022. Europe PMC, https://doi.org/10.1101/2022.06.07.495149.
Frank, Matthew G., et al. “SARS-CoV-2 Spike S1 Subunit Induces Neuroinflammatory, Microglial and Behavioral Sickness Responses: Evidence of PAMP-like Properties.” Brain, Behavior, and Immunity, vol. 100, Feb. 2022, pp. 267–77. PubMed Central, https://doi.org/10.1016/j.bbi.2021.12.007.
Gloger, Elana M., and Suzanne C. Segerstrom. “Repetitive Thought, Cognition, and Systemic Inflammation in the Midlife in the United States Study.” Psychology & Health, June 2022, pp. 1–19. PubMed, https://doi.org/10.1080/08870446.2022.2092104.
Grobbelaar, Lize M., et al. “SARS-CoV-2 Spike Protein S1 Induces Fibrin(Ogen) Resistant to Fibrinolysis: Implications for Microclot Formation in COVID-19.” Bioscience Reports, vol. 41, no. 8, Aug. 2021, p. BSR20210611. PubMed Central, https://doi.org/10.1042/BSR20210611.
Hennessy, Edel, et al. “Systemic TNF-α Produces Acute Cognitive Dysfunction and Exaggerated Sickness Behavior When Superimposed upon Progressive Neurodegeneration.” Brain, Behavior, and Immunity, vol. 59, Jan. 2017, pp. 233–44. PubMed Central, https://doi.org/10.1016/j.bbi.2016.09.011.
Hwang, Eun Seong, and Seon Beom Song. “Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment.” Biomolecules, vol. 10, no. 5, Apr. 2020, p. E687. PubMed, https://doi.org/10.3390/biom10050687.
Kell, Douglas B., et al. “A Central Role for Amyloid Fibrin Microclots in Long COVID/PASC: Origins and Therapeutic Implications.” Biochemical Journal, vol. 479, no. 4, Feb. 2022, pp. 537–59. PubMed Central, https://doi.org/10.1042/BCJ20220016.
Khuu, Maggie A., et al. “Stage-Dependent Effects of Intermittent Hypoxia Influence the Outcome of Hippocampal Adult Neurogenesis.” Scientific Reports, vol. 11, Mar. 2021, p. 6005. PubMed Central, https://doi.org/10.1038/s41598-021-85357-5.
Krapić, Mia, et al. “Immunological Mechanisms of Sickness Behavior in Viral Infection.” Viruses, vol. 13, no. 11, Nov. 2021, p. 2245. PubMed Central, https://doi.org/10.3390/v13112245.
Krishnan, Kamini, et al. “Multidisciplinary Approach to Brain Fog and Related Persisting Symptoms Post COVID-19.” Journal of Health Service Psychology, vol. 48, no. 1, 2022, pp. 31–38. PubMed Central, https://doi.org/10.1007/s42843-022-00056-7.
Li, Zhichao, et al. “Neuroinflammation as the Underlying Mechanism of Postoperative Cognitive Dysfunction and Therapeutic Strategies.” Frontiers in Cellular Neuroscience, vol. 16, Mar. 2022, p. 843069. PubMed Central, https://doi.org/10.3389/fncel.2022.843069.
Nieraad, Hendrik, et al. “Hyperhomocysteinemia: Metabolic Role and Animal Studies with a Focus on Cognitive Performance and Decline—A Review.” Biomolecules, vol. 11, no. 10, Oct. 2021, p. 1546. PubMed Central, https://doi.org/10.3390/biom11101546.
Ocon, Anthony J. “Caught in the Thickness of Brain Fog: Exploring the Cognitive Symptoms of Chronic Fatigue Syndrome.” Frontiers in Physiology, vol. 4, Apr. 2013, p. 63. PubMed Central, https://doi.org/10.3389/fphys.2013.00063.
Ogoh, Shigehiko. “Relationship between Cognitive Function and Regulation of Cerebral Blood Flow.” The Journal of Physiological Sciences, vol. 67, no. 3, May 2017, pp. 345–51. jps.biomedcentral.com, https://doi.org/10.1007/s12576-017-0525-0.
Olshansky, Brian, et al. “Postural Orthostatic Tachycardia Syndrome (POTS): A Critical Assessment.” Progress in Cardiovascular Diseases, vol. 63, no. 3, 2020, pp. 263–70. PubMed Central, https://doi.org/10.1016/j.pcad.2020.03.010.
Peeri, Noah C., et al. “Association of Magnesium Intake and Vitamin D Status with Cognitive Function in Older Adults: An Analysis of US National Health and Nutrition Examination Survey (NHANES) 2011 to 2014.” European Journal of Nutrition, vol. 60, no. 1, Feb. 2021, pp. 465–74. PubMed Central, https://doi.org/10.1007/s00394-020-02267-4.
Pretorius, Etheresia, et al. “Persistent Clotting Protein Pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Is Accompanied by Increased Levels of Antiplasmin.” Cardiovascular Diabetology, vol. 20, no. 1, Aug. 2021, p. 172. BioMed Central, https://doi.org/10.1186/s12933-021-01359-7.
Raj, Vidya, et al. “Cognitive and Psychological Issues in Postural Tachycardia Syndrome.” Autonomic Neuroscience : Basic & Clinical, vol. 215, Dec. 2018, pp. 46–55. PubMed Central, https://doi.org/10.1016/j.autneu.2018.03.004.
Reynolds, E. H. “Folic Acid, Ageing, Depression, and Dementia.” BMJ : British Medical Journal, vol. 324, no. 7352, June 2002, pp. 1512–15. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1123448/.
Ryu, Jae Kyu, et al. SARS-CoV-2 Spike Protein Induces Abnormal Inflammatory Blood Clots Neutralized by Fibrin Immunotherapy. bioRxiv, 13 Oct. 2021, p. 2021.10.12.464152. bioRxiv, https://www.biorxiv.org/content/10.1101/2021.10.12.464152v1.
Santangelo, Gabriella, et al. “Neuropsychological Profile in Parents of Adult Phenylketonuria Patients.” Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, vol. 39, no. 1, Jan. 2018, pp. 161–64. PubMed, https://doi.org/10.1007/s10072-017-3181-5.
Seet, Dominic, et al. “Cognitive Dysfunction in Systemic Lupus Erythematosus: Immunopathology, Clinical Manifestations, Neuroimaging and Management.” Rheumatology and Therapy, vol. 8, no. 2, May 2021, pp. 651–79. PubMed Central, https://doi.org/10.1007/s40744-021-00312-0.
Singh, Satish, et al. “Alpha2-Antiplasmin: The Devil You Don’t Know in Cerebrovascular and Cardiovascular Disease.” Frontiers in Cardiovascular Medicine, vol. 7, Dec. 2020, p. 608899. PubMed Central, https://doi.org/10.3389/fcvm.2020.608899.
Sklinda, Katarzyna, et al. “Ischaemic Background of Brain Fog in Long-Haul COVID-19 – a Nuclear Magnetic Resonance Spectroscopy-Based Metabonomic Analysis. Preliminary Results.” Polish Journal of Radiology, vol. 86, Dec. 2021, pp. e654–60. PubMed Central, https://doi.org/10.5114/pjr.2021.111100.
Smith, Taryn J., et al. “Thiamine Deficiency Disorders: A Clinical Perspective.” Annals of the New York Academy of Sciences, vol. 1498, no. 1, Aug. 2021, pp. 9–28. PubMed Central, https://doi.org/10.1111/nyas.14536.
Tay, Sen Hee, et al. “25-Hydroxyvitamin D3 Deficiency Independently Predicts Cognitive Impairment in Patients with Systemic Lupus Erythematosus.” PLoS ONE, vol. 10, no. 12, Dec. 2015, p. e0144149. PubMed Central, https://doi.org/10.1371/journal.pone.0144149.
Wei, Jiaqi, et al. “The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis.” Frontiers in Neurology, vol. 11, Dec. 2020, p. 581346. PubMed Central, https://doi.org/10.3389/fneur.2020.581346.
Yelland, Gregory W. “Gluten-Induced Cognitive Impairment (‘Brain Fog’) in Coeliac Disease: Cognitive Impairment in Coeliac Disease.” Journal of Gastroenterology and Hepatology, vol. 32, Mar. 2017, pp. 90–93. DOI.org (Crossref), https://doi.org/10.1111/jgh.13706.
Yoshikawa, Takeo, et al. “Histamine N-Methyltransferase in the Brain.” International Journal of Molecular Sciences, vol. 20, no. 3, Feb. 2019, p. 737. PubMed Central, https://doi.org/10.3390/ijms20030737.