Key Takeaways:
~ Dental implants can replace a lost tooth, filling the gap and preventing bone loss.
~ The published failure rate of dental implants is fairly low, but the rate of peri-implantitis, or inflammation in the implant area, is about 1 in 3.
~ Genetic variants can have a surprisingly large impact on the likelihood of peri-implantitis and the long-term success of your dental implant.
~ Importantly, there are natural ways to minimize the risk of peri-implantitis and implant failure.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.
Dental Implants:
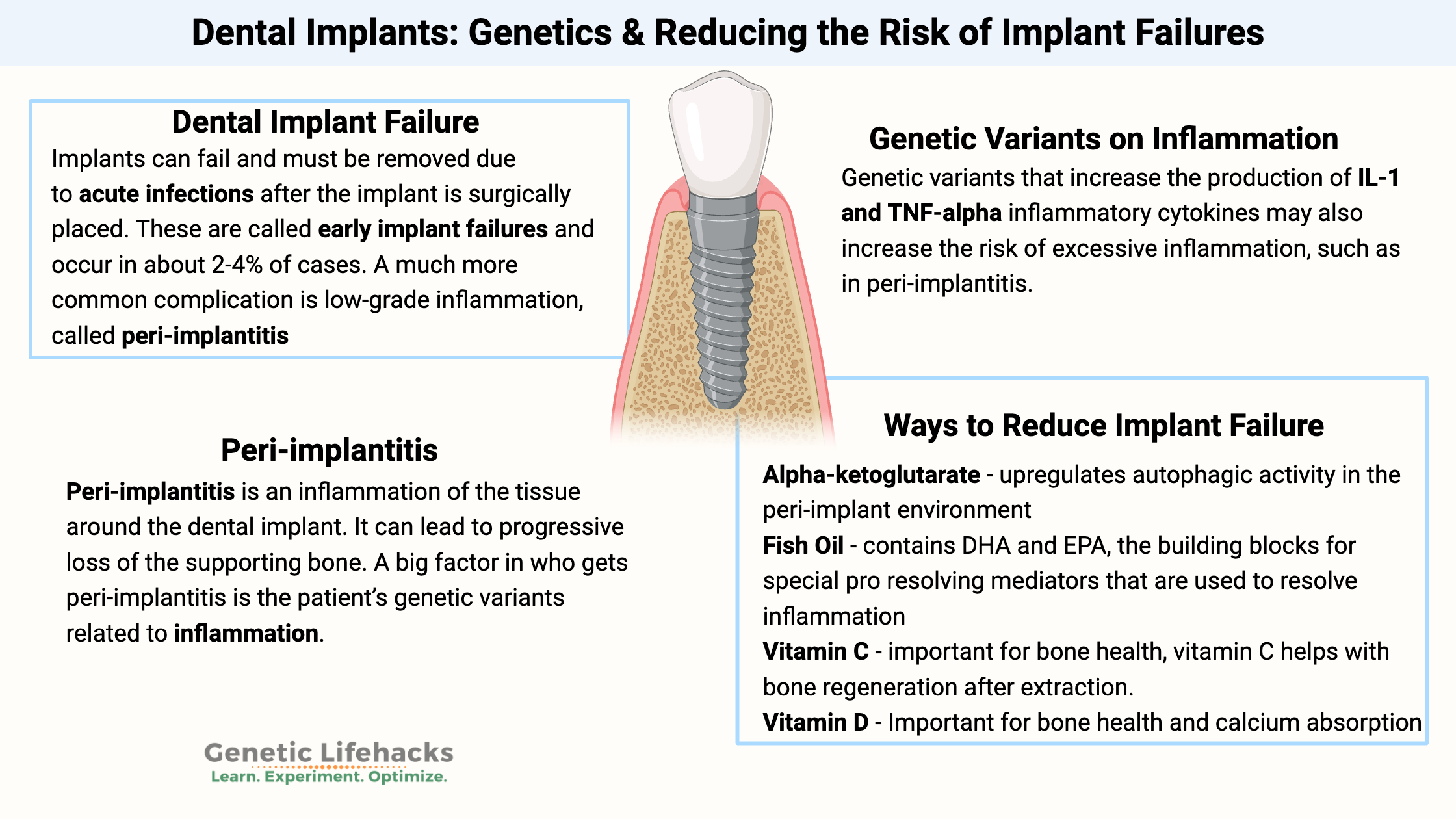
A dental implant replaces a lost tooth with a post screwed into the jawbone and a crown. It’s a long process that starts with the removal of an infected or damaged tooth, followed months later with the implant. Often a bone graft is used to restore the bone where the tooth was extracted.
The dental implant market in the U.S. is large – approximately $1.1 billion/year in 2020. A common reason for needing implants is a failed root canal.[ref]
Bone grafts: After a tooth is removed, the gap in the bone left by the removal of the root needs to be filled. Bone grafting is often used to build new bone in the area where the tooth was removed and to prevent further bone loss or resorption. Talk to your dentist or oral surgeon about the different materials available for bone grafting.
Dental Implant Failure:
The 10-year failure rate of implants is 1.9-3.6%. One lifestyle factor that increases the failure rate is smoking. Chronic medical problems, such as diabetes or alcoholism, can also increase the likelihood of failure.[ref][ref][ref]
Implants can fail and must be removed due to acute infections after the implant is surgically placed. These are called early implant failures and occur in about 2-4% of cases.[ref]
A much more common complication is low-grade inflammation, called peri-implantitis, which can eventually lead to bone resorption and loss of the implant.
Peri-implantitis doesn’t usually require immediate removal of the implant. Instead, it is a long-term problem that reduces the longevity of the implant.
Peri-implantitis: Understanding the Causes to Prevent It
Peri-implantitis is an inflammation of the tissue around the dental implant. It can lead to progressive loss of the supporting bone. A study of more than 6,000 implants found that the average incidence of peri-implantitis was 34% over two years. Periodontitis increased the risk of peri-implantitis by 3.6 times. However, using antibiotics in conjunction with implant surgery reduced the risk by 80%.[ref]
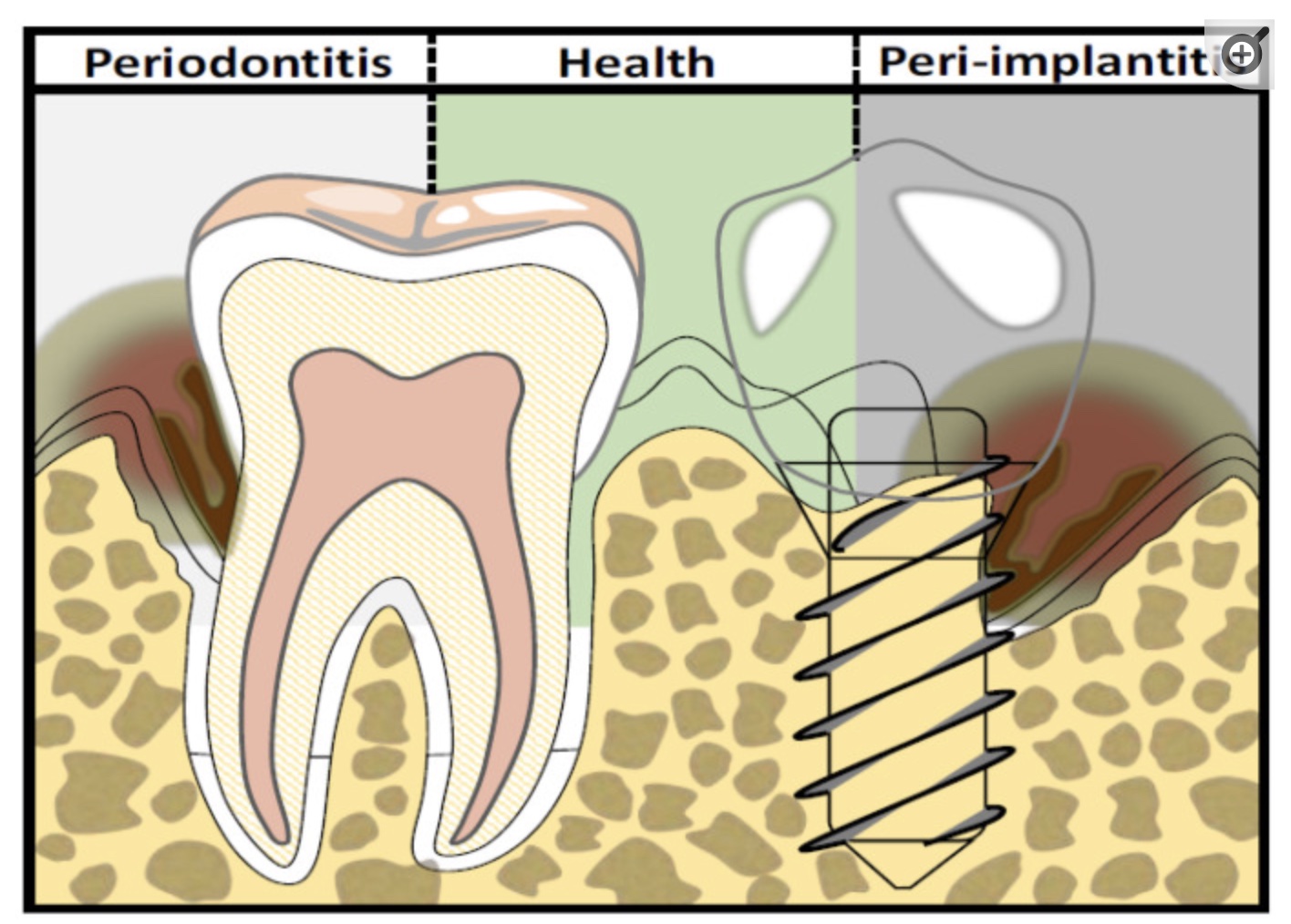
Other studies put the prevalence of peri-implantitis at ~20%. The variability in studies depends on the follow-up period (months vs. years) and how the disease is defined.[ref]
What causes peri-implantitis?
- A poorly placed implant increases the risk of peri-implantitis.
- The breakdown of the titanium in the implant can also increase inflammation in the surrounding tissue.[ref]
- Smoking and diabetes increase inflammation throughout the body and also increase the risk of peri-implantitis.[ref]
One study found that over half of all the implant failures were clustered in only one-third of patients. This suggests an individual susceptibility to failure.[ref]
Our genomes show many individual differences in the genes of the immune system. It’s a huge advantage for the overall success of a species to have different parts of the population able to fight off different types of pathogens. This makes it less likely that a new pathogen will wipe out the entire population.
Part of your immune response is the production of inflammatory cytokines. Take TNF-alpha for example: The TNF-alpha cytokine is great when you’re fighting off leprosy, but the overproduction of TNF-alpha can lead to excessive and chronic inflammation.
A big factor in who gets peri-implantitis is the patient’s genetic variants related to inflammation. Not surprisingly, these genes also overlap with the risk of periodontitis.[ref]
Genetic influences on implant inflammation:
The extraction, bone regeneration, and implantation process all trigger macrophages to release inflammatory cytokines, such as interleukin 1 and TNF-alpha.
Interleukin 1 (IL-1) and TNF-alpha are absolutely necessary at the correct physiological levels after tooth extraction. Both are needed to trigger bone formation and the subsequent integration of the implant.
However, genetic variants that increase the production of IL-1 and TNF-alpha cytokines may also increase the risk of excessive inflammation, such as in peri-implantitis.
Breakdown of the implant material:
Titanium is the most commonly used metal for implants. A number of factors come together in the mouth to affect the longevity of the titanium. The pH of the saliva (due to bacterial load), fluoride, biofilm, and chewing force can all come together to break down the outer layer of the titanium implant. The breakdown of titanium can lead to inflammation around the implant as well as increased RANKL (an immune system protein that regulates bone breakdown). Excessive RANKL can decrease bone formation.[ref]
Systemic allergies to titanium can also develop, although this is uncommon. In addition to allergy-like symptoms such as eczema, redness, and swelling, one case study reports implant failure due to titanium allergy.[ref][ref][ref]
More on this, along with other options, such as zirconia, can be found in the Lifehacks section below.
Healing after tooth removal and implant surgery:
There is a complex process of healing and restoring the tissue for any wound.
Inflammation is a natural response to an injury or wound. The damaged tissue releases chemical signals that prompt a cascade of immune system reactions to occur. For example, blood vessels dilate to increase blood flow to the area, and white blood cells are recruited to the site to remove damaged cells and bacteria.
What is often not discussed, though, is that the resolution of inflammation is also an active process that is an essential part of wound healing and tissue repair.
The resolution of inflammation involves lipid signaling molecules called specialized pro‐resolving mediators (SPMs). These lipids are essential in initiating healing, recruiting stem cells, and then stopping the inflammatory cytokines.
Specialized pro-resolving mediators, SPMs, are synthesized by cells using DHA and EPA, which are omega-3 fatty acids found in fish oil. SPMs needed for resolving inflammation after dental surgery include lipoxins, resolvins, and protectins.[ref]
Macrophages are a type of white blood cell that can help clear pathogens or cellular debris from a wound. There are two types of macrophages – a pro-inflammatory and an anti-inflammatory subtype. SPMs help to move the macrophages from the initial pro-inflammatory activation type to the anti-inflammatory phenotype needed for wound healing.
Specialized stem cells and fibroblasts that form connective tissue are needed to regenerate the bone and heal after surgery. SPMs, specifically resolvins, are essential in this process.
Animal studies show how resolvins and lipoxins, two types of SPMs, are essential for healing periodontitis lesions and restoring tissue homeostasis. Essentially, these SPMs promote tissue regeneration in the wound and protect the periodontal ligament from chronic inflammation.[ref]
Dental Implants Genotype Report:
Access this content:
An active subscription is required to access this content.
Lifehacks: Natural Solutions for Improving Implant Success
Preventing the need for an implant:
The pulp in a tooth’s root can become infected and lead to a root canal. Tooth pulp is also a great source of stem cells. A recent study explored using CBD oil to promote dental pulp stem cell proliferation, migration, and osteogenic differentiation. The study found that CBD oil downregulated TNF-alpha to a level where inflammation no longer inhibited the stem cells’ potential for regeneration. The study is open access if you want to read it for more details.[ref]
Titanium vs. Zirconia Implants
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
Fluoride: Understanding Its Effects on Health
Discover the pros and cons of fluoride for dental health and overall wellness, its sources, toxicity levels, and how genetics influence fluoride sensitivity.
Are your cavities caused by genetics?
It turns out that genetics plays a larger role here than you would think. Researchers estimate that the ‘heritability’ or genetic component of dental caries is about 50%.
Chronic Sinus Infections: Genetics, Reasons, Solutions
Genetics plays a role in the likelihood of having chronic sinus problems. This article looks at the genetic reasons driving some people to have chronic sinus infections.
Genomics and Teeth Grinding (Bruxism)
Grinding your teeth at night can be due, in part, to genetic variants in the serotonin genes.
References:
Agrawal, Kaushal Kishor, et al. “Association of Interleukin-1 Gene Polymorphism and Early Crestal Bone Loss around Submerged Dental Implants: A Systematic Review and Meta-Analysis.” Journal of Indian Prosthodontic Society, vol. 21, no. 2, 2021, pp. 116–24. PubMed, https://doi.org/10.4103/jips.jips_511_20.
Ali, Muhanad, et al. “Application of Specialized Pro‐resolving Mediators in Periodontitis and Peri‐implantitis: A Review.” European Journal of Oral Sciences, vol. 129, no. 1, Feb. 2021, p. e12759. PubMed Central, https://doi.org/10.1111/eos.12759.
Al-Obaidi, Mazen M. Jamil, Fouad Hussain Al-Bayaty, Rami Al Batran, Jamal Hussaini, et al. “Impact of Ellagic Acid in Bone Formation after Tooth Extraction: An Experimental Study on Diabetic Rats.” TheScientificWorldJournal, vol. 2014, 2014, p. 908098. PubMed, https://doi.org/10.1155/2014/908098.
Al-Obaidi, Mazen M. Jamil, Fouad Hussain Al-Bayaty, Rami Al Batran, Pouya Hassandarvish, et al. “Protective Effect of Ellagic Acid on Healing Alveolar Bone after Tooth Extraction in Rat–a Histological and Immunohistochemical Study.” Archives of Oral Biology, vol. 59, no. 9, Sept. 2014, pp. 987–99. PubMed, https://doi.org/10.1016/j.archoralbio.2014.06.001.
Baró, María A., et al. “Alveolar Wound Healing in Rats Fed on High Sucrose Diet.” Acta Odontologica Latinoamericana: AOL, vol. 26, no. 2, 2013, pp. 97–103.
Camps-Font, Octavi, et al. “Postoperative Infections after Dental Implant Placement: Variables Associated with Increased Risk of Failure.” Journal of Periodontology, vol. 89, no. 10, Oct. 2018, pp. 1165–73. PubMed, https://doi.org/10.1002/JPER.18-0024.
Consultant, Dental. “Dental Implant Statistics 2022-2021, 2020 | Implant Facts and Data Trends 2019.” 2740 Consulting, 24 Aug. 2020, https://www.2740consulting.com/dental-implant-statistics/.
Corrêa, Monica Grazieli, et al. “Impact of Resveratrol in the Reduction of the Harmful Effect of Diabetes on Peri-Implant Bone Repair: Bone-Related Gene Expression, Counter-Torque and Micro-CT Analysis in Rats.” Acta Odontologica Scandinavica, vol. 79, no. 3, Apr. 2021, pp. 174–81. PubMed, https://doi.org/10.1080/00016357.2020.1797159.
de Lima-Souza, Reydson Alcides, et al. “Multiple Cutaneous Fistula after Titanium Dental Implant: A Case Report.” Clinical Implant Dentistry and Related Research, vol. 23, no. 2, Apr. 2021, pp. 270–74. PubMed, https://doi.org/10.1111/cid.12972.
De Waal, Yvonne C. M., et al. “Systemic Antibiotic Therapy as an Adjunct to Non-Surgical Peri-Implantitis Treatment: A Single-Blind RCT.” Journal of Clinical Periodontology, vol. 48, no. 7, July 2021, pp. 996–1006. PubMed, https://doi.org/10.1111/jcpe.13464.
Diaz, Pedro, et al. “What Is the Prevalence of Peri-Implantitis? A Systematic Review and Meta-Analysis.” BMC Oral Health, vol. 22, no. 1, Oct. 2022, p. 449. PubMed, https://doi.org/10.1186/s12903-022-02493-8.
Domínguez-Pérez, Rubén Abraham, et al. “Association of Cytokines Polymorphisms with Chronic Peridontitis and Rheumatoid Arthritis in a Mexican Population.” Acta Odontologica Scandinavica, vol. 75, no. 4, May 2017, pp. 243–48. PubMed, https://doi.org/10.1080/00016357.2017.1280846.
Dominici, Roberto, et al. “Cloning and Functional Analysis of the Allelic Polymorphism in the Transcription Regulatory Region of Interleukin-1 Alpha.” Immunogenetics, vol. 54, no. 2, May 2002, pp. 82–86. PubMed, https://doi.org/10.1007/s00251-002-0445-9.
Dudics, Steven, et al. “Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome.” International Journal of Molecular Sciences, vol. 19, no. 9, Sept. 2018, p. 2508. www.mdpi.com, https://doi.org/10.3390/ijms19092508.
Elnagdy, Shorouk, et al. “Local Oral Delivery Agents with Anti-Biofilm Properties for the Treatment of Periodontitis and Peri-Implantitis. A Narrative Review.” Molecules, vol. 26, no. 18, Sept. 2021, p. 5661. PubMed Central, https://doi.org/10.3390/molecules26185661.
Feng, Bo, et al. “Association of Tumor Necrosis Factor α -308G/A and Interleukin-6 -174G/C Gene Polymorphism with Pneumonia-Induced Sepsis.” Journal of Critical Care, vol. 30, no. 5, Oct. 2015, pp. 920–23. PubMed, https://doi.org/10.1016/j.jcrc.2015.04.123.
Gaur, Sumit, et al. “Bio-Tribocorrosion of Titanium Dental Implants and Its Toxicological Implications: A Scoping Review.” The Scientific World Journal, vol. 2022, Oct. 2022, p. 4498613. PubMed Central, https://doi.org/10.1155/2022/4498613.
Harrel, Stephen K., et al. “The Potential Role of Titanium Allergy in Implant Failure.” The International Journal of Periodontics & Restorative Dentistry, vol. 42, no. 6, 2022, pp. 783–87. PubMed, https://doi.org/10.11607/prd.6206.
Hong, Seoung-Jin, et al. “A Novel Retentive Type of Dental Implant Prosthesis: Marginal Fitness of the Cementless Double Crown Type Implant Prosthesis Evaluated by Bacterial Penetration and Viability.” The Journal of Advanced Prosthodontics, vol. 12, no. 4, Aug. 2020, pp. 233–38. PubMed Central, https://doi.org/10.4047/jap.2020.12.4.233.
Irastorza, I., et al. “Adhesion, Integration and Osteogenesis of Human Dental Pulp Stem Cells on Biomimetic Implant Surfaces Combined with Plasma Derived Products.” European Cells & Materials, vol. 38, Nov. 2019, pp. 201–14. PubMed, https://doi.org/10.22203/eCM.v038a14.
Irshad, Muhammad, et al. “Influence of Titanium on in Vitro Fibroblast- Porphyromonas Gingivalis Interaction in Peri-Implantitis.” Journal of Clinical Periodontology, vol. 40, no. 9, Sept. 2013, pp. 841–49. DOI.org (Crossref), https://doi.org/10.1111/jcpe.12136.
Iviglia, Giorgio, and Marco Morra. “Engineering Interfacial Environment of Epigallocatechin Gallate Coated Titanium for Next-Generation Bioactive Dental Implant Components.” International Journal of Molecular Sciences, vol. 24, no. 3, Jan. 2023, p. 2661. PubMed Central, https://doi.org/10.3390/ijms24032661.
Jiménez-Sousa, María Ángeles, et al. “IL-1B Rs16944 Polymorphism Is Related to Septic Shock and Death.” European Journal of Clinical Investigation, vol. 47, no. 1, Jan. 2017, pp. 53–62. PubMed, https://doi.org/10.1111/eci.12702.
Jin, Qiuchen, et al. “Association between Common Polymorphisms in IL-1 and TNFα and Risk of Peri-Implant Disease: A Meta-Analysis.” PLoS ONE, vol. 16, no. 10, Oct. 2021, p. e0258138. PubMed Central, https://doi.org/10.1371/journal.pone.0258138.
Laleman, Isabelle, et al. “The Usage of a Lactobacilli Probiotic in the Non-Surgical Therapy of Peri-Implantitis: A Randomized Pilot Study.” Clinical Oral Implants Research, vol. 31, no. 1, Jan. 2020, pp. 84–92. PubMed, https://doi.org/10.1111/clr.13555.
Lang, N. P., et al. “Effect of Interleukin-1 Gene Polymorphisms on Gingival Inflammation Assessed by Bleeding on Probing in a Periodontal Maintenance Population.” Journal of Periodontal Research, vol. 35, no. 2, Apr. 2000, pp. 102–07. PubMed, https://doi.org/10.1034/j.1600-0765.2000.035002102.x.
Li, Xiao, et al. “Role of Vitamin C in Wound Healing after Dental Implant Surgery in Patients Treated with Bone Grafts and Patients with Chronic Periodontitis.” Clinical Implant Dentistry and Related Research, vol. 20, no. 5, Oct. 2018, pp. 793–98. PubMed, https://doi.org/10.1111/cid.12647.
Liu, Ruojing, et al. “Alpha-Ketoglutarate up-Regulates Autophagic Activity in Peri-Implant Environment and Enhances Dental Implant Osseointegration in Osteoporotic Mice.” Journal of Clinical Periodontology, vol. 50, no. 5, May 2023, pp. 671–83. PubMed, https://doi.org/10.1111/jcpe.13784.
Majumder, Poulami, et al. “Interleukin Gene Polymorphisms in Chronic Periodontitis: A Case-Control Study in the Indian Population.” Archives of Oral Biology, vol. 101, May 2019, pp. 156–64. PubMed, https://doi.org/10.1016/j.archoralbio.2019.03.015.
Meyle, Joerg, et al. “General Genetic and Acquired Risk Factors, and Prevalence of Peri-Implant Diseases – Consensus Report of Working Group 1.” International Dental Journal, vol. 69, Sept. 2019, pp. 3–6. DOI.org (Crossref), https://doi.org/10.1111/idj.12489.
Nakano, Ryotaro, et al. “Influence of Eccentric Cyclic Loading on Implant Components: Comparison between Titanium and Zirconia Abutments.” Dental Materials Journal, vol. 40, no. 1, Jan. 2021, pp. 235–44. PubMed, https://doi.org/10.4012/dmj.2020-030.
Nastri, Livia, et al. “Do Dietary Supplements and Nutraceuticals Have Effects on Dental Implant Osseointegration? A Scoping Review.” Nutrients, vol. 12, no. 1, Jan. 2020, p. 268. PubMed Central, https://doi.org/10.3390/nu12010268.
Petkovic-Curcin, Aleksandra, et al. “Association of Cytokine Gene Polymorphism with Peri-Implantitis Risk.” The International Journal of Oral & Maxillofacial Implants, vol. 32, no. 5, 2017, pp. e241–48. PubMed, https://doi.org/10.11607/jomi.5814.
Picos, Andrei, et al. “Interleukin-1A and Interleukin-1B Gene Polymorphisms in Gastroesophageal Reflux Disease.” Experimental and Therapeutic Medicine, vol. 20, no. 4, Oct. 2020, pp. 3394–98. PubMed Central, https://doi.org/10.3892/etm.2020.9030.
Pigossi, Suzane C., et al. “Association of Interleukin 4 Gene Polymorphisms with Dental Implant Loss.” Implant Dentistry, vol. 23, no. 6, Dec. 2014, pp. 723–31. PubMed, https://doi.org/10.1097/ID.0000000000000157.
Quesada-García, Ma Pilar, et al. “Dental Implant Stability Is Influenced by Implant Diameter and Localization and by the Use of Plasma Rich in Growth Factors.” Journal of Oral and Maxillofacial Surgery: Official Journal of the American Association of Oral and Maxillofacial Surgeons, vol. 70, no. 12, Dec. 2012, pp. 2761–67. PubMed, https://doi.org/10.1016/j.joms.2012.08.006.
Rakic, Mia, et al. “CD14 and TNFα Single Nucleotide Polymorphisms Are Candidates for Genetic Biomarkers of Peri-Implantitis.” Clinical Oral Investigations, vol. 19, no. 4, May 2015, pp. 791–801. Springer Link, https://doi.org/10.1007/s00784-014-1313-3.
Ribeiro, Fernanda Vieira, et al. “Resveratrol Reverses the Negative Effect of Smoking on Peri-Implant Repair in the Tibia of Rats.” Clinical Oral Implants Research, vol. 30, no. 1, Jan. 2019, pp. 1–10. PubMed, https://doi.org/10.1111/clr.13384.
Rong, Hao, et al. “Association between IL1B Polymorphisms and the Risk of Rheumatoid Arthritis.” International Immunopharmacology, vol. 83, June 2020, p. 106401. PubMed, https://doi.org/10.1016/j.intimp.2020.106401.
Schulz, Susanne, et al. “Single Nucleotide Polymorphisms in Interleukin-1gene Cluster and Subgingival Colonization with Aggregatibacter Actinomycetemcomitans in Patients with Aggressive Periodontitis.” Human Immunology, vol. 72, no. 10, Oct. 2011, pp. 940–46. PubMed, https://doi.org/10.1016/j.humimm.2011.05.009.
Schwarz, Frank, et al. “Peri-Implantitis.” Journal of Periodontology, vol. 89 Suppl 1, June 2018, pp. S267–90. PubMed, https://doi.org/10.1002/JPER.16-0350.
Shah, Shahid Ahmad, et al. “Biological and Esthetic Outcome of Immediate Dental Implant with the Adjunct Pretreatment of Immediate Implants with Platelet-Rich Plasma or Photofunctionalization: A Randomized Controlled Trial.” Journal of Indian Prosthodontic Society, vol. 21, no. 4, 2021, pp. 348–55. PubMed, https://doi.org/10.4103/jips.jips_217_21.
Sivaraman, Karthik, et al. “Is Zirconia a Viable Alternative to Titanium for Oral Implant? A Critical Review.” Journal of Prosthodontic Research, vol. 62, no. 2, Apr. 2018, pp. 121–33. PubMed, https://doi.org/10.1016/j.jpor.2017.07.003.
Tavares, M., et al. “Tumour Necrosis Factor-Alpha (-308G/A) Promoter Polymorphism Is Associated with Ulcerative Colitis in Brazilian Patients.” International Journal of Immunogenetics, vol. 43, no. 6, Dec. 2016, pp. 376–82. PubMed, https://doi.org/10.1111/iji.12289.
Werny, Joscha G., et al. “Does Vitamin D Have an Effect on Osseointegration of Dental Implants? A Systematic Review.” International Journal of Implant Dentistry, vol. 8, Apr. 2022, p. 16. PubMed Central, https://doi.org/10.1186/s40729-022-00414-6.
Wu, Xiangbing, et al. “Melatonin Prevents Peri‑implantitis via Suppression of TLR4/NF-ΚB.” Acta Biomaterialia, vol. 134, Oct. 2021, pp. 325–36. PubMed, https://doi.org/10.1016/j.actbio.2021.07.017.
Yu, Lina, et al. “Cannabidiol Rescues TNF-α-Inhibited Proliferation, Migration, and Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells.” Biomolecules, vol. 13, no. 1, Jan. 2023, p. 118. PubMed, https://doi.org/10.3390/biom13010118.
Yucesoy, Berran, et al. “Genetic Variants in TNFα, TGFB1, PTGS1 and PTGS2 Genes Are Associated with Diisocyanate-Induced Asthma.” Journal of Immunotoxicology, vol. 13, no. 1, 2016, pp. 119–26. PubMed, https://doi.org/10.3109/1547691X.2015.1017061.