Key takeaways:
~ Dry eyes can have multiple causes – from increased osmolality to elevated inflammatory response. Vitamin deficiencies, or more specifically, a deficiency of the right form of a vitamin, can also cause dry eyes.
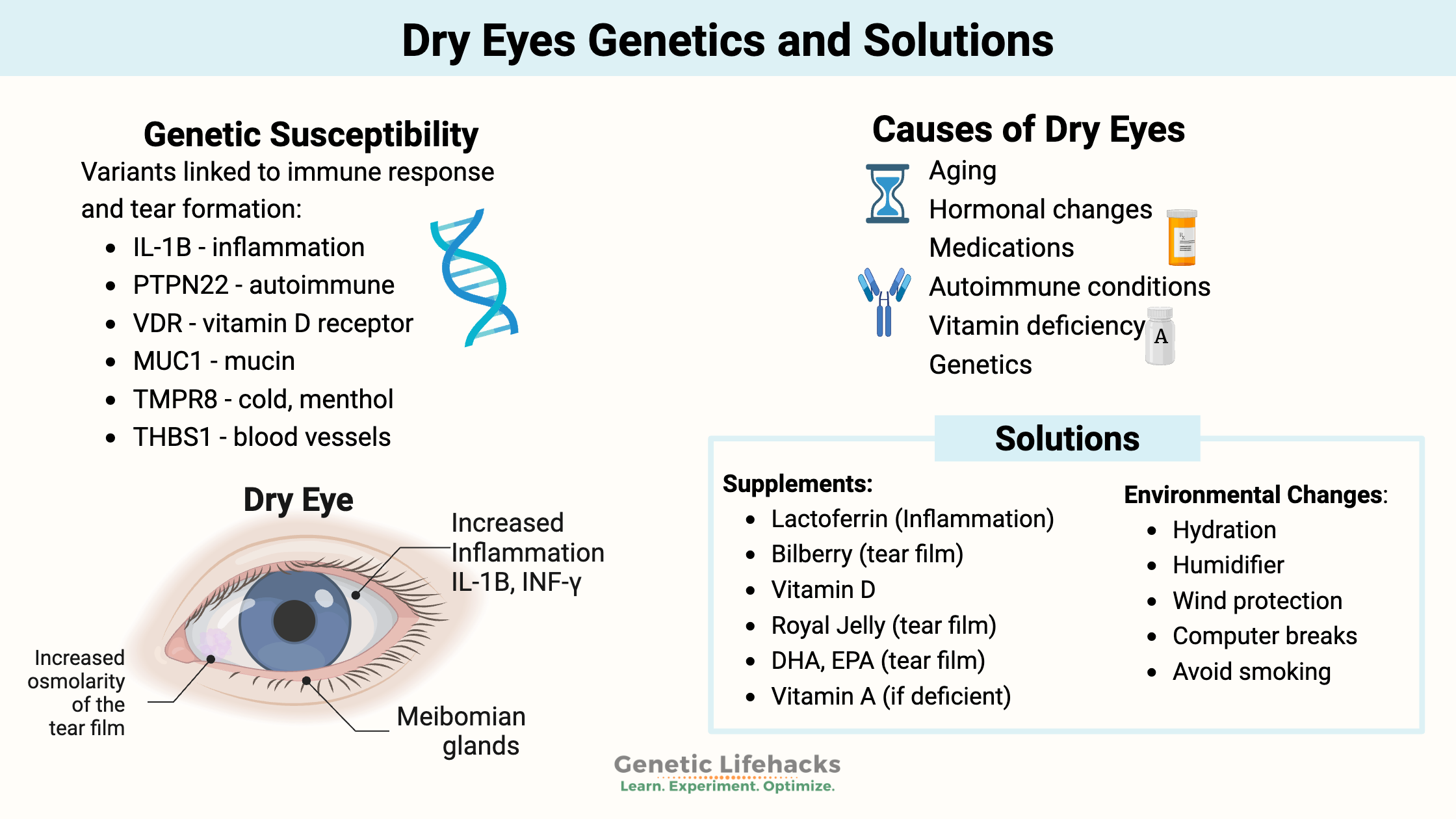
~ Multiple genetic variants are associated with increased susceptibility to dry eyes.
~ Understanding the pathways involved and your genetic susceptibility can help you target the right solution.
~ Find out which dry eye treatment options have research to back them – and learn how they interact with your genes.
Dry Eyes: Root Causes, Genetic Variants, and Real Solutions
I spend a lot of time on the computer, in a dry climate, at a somewhat high altitude… Montana is a beautiful place to live, but it is killing my eyes! Worst of all, my vision seems to be periodically blurry (especially at night).
My goal? To find out the underlying reason for my dry eyes, and then solve the issue for good. Along the way, I’m going to dive into how tears work, why eye moisture is so important, how to get rid of blurred vision, and the genetic drivers of dry eyes.
Dry eye disease (DED) is characterized by “eye dryness, discomfort, and sensitivity to light.”[ref] Blurred vision can also occur due to dry eyes, and a halo effect for lights at night is another common problem.
Multiple causes of dry eyes:
In general, typical health websites will tell you that there are a couple of reasons for dry eyes:
1) Not enough tears are produced because:
- getting old (tear production reduced over 50)
- autoimmune diseases
- using medications (antihistamines, hormone replacement therapy, antidepressants, birth control)
– OR –
2) Poor-quality tears due to:
- Hormone changes (in aging)
- autoimmune diseases
- inflamed eyelid glands
Essentially, the standard answer seems to be hormones or age, suggesting that there isn’t much to be done. Let’s dig into the actual root causes because “you have dry eyes because you are getting old” just doesn’t cut it for me.
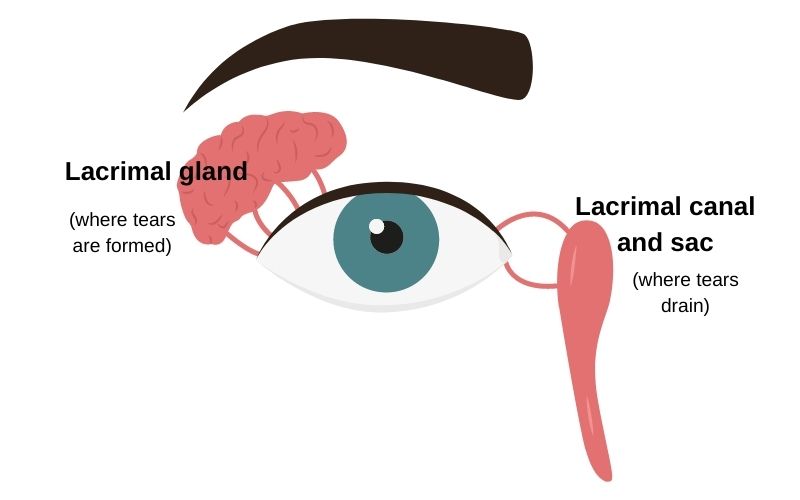
Before we get started, here’s a quick image showing where tears form and where they drain out:
What is happening in dry eyes:
Essentially, the latest research shows that dry eye disease can be due to dysregulation of the ocular (eye) surface. It can occur due to surface immune responses.[ref]
Two things seem to happen at the same time:[ref]
- increased osmolarity of the tear film (osmolarity is the measure of solute concentration)
- inflammation of the ocular surface
Let’s tackle inflammation first:
Inflammation of the surface of the eye
In people with Sjogren’s syndrome, an autoimmune condition, researchers found T-cell infiltration (immune cells on the eye surface), increased expression of HLA-DR, and IFNG mRNA.[ref] This means there is active inflammation on the surface of the eye.
Related article: Genetics and the causes of Sjogren’s Syndrome
While dry eyes are a hallmark of Sjogren’s syndrome, other researchers have found that people without the autoimmune disease also have elevated T-cell infiltration of the ocular surface with dry eye disease.[ref]
Researchers have also found that HLA-DR expression increases in dry eye disease. The HLA system is part of the immune system and marks cells to be identified as ‘self’ or ‘foreign’. Some studies show that the increase in HLA-DR expression is up to 4 to 6-fold, which is very significant. It indicates that inflammation occurs on the eye’s surface cells during dry eye disease.[ref]
HMGB1 is a protein that signals for inflammation. It is released when cells are damaged or by activated immune cells. Researchers find that HMGB1 is elevated in dry eye disease.[ref] Animal studies show that anti-HMGB1 molecules can attenuate dry eye disease.[ref] Interestingly, exposure to low-humidity environments worsens dry eyes and also increases HMGB1 levels.[ref]
To recap: Inflammatory cytokines are elevated on the eye surface in dry eyes.
Tear film disruption: keeping the eye moist
Tears are a lot more complicated than just ‘salty water’. It is a complex solution that has to have the right fluidity and viscosity to coat the eye and keep it at the right moisture. Tears contain lipids, mucus, and an ion solution that all work together.
Inflammatory cytokines alter the production of mucus, which is a component of tears that helps keep tears from evaporating too quickly. The inflammatory cytokines found here include IL-1B (interleukin 1 beta) and IFN-γ (interferon-gamma).
The meibomian glands are along the rim of the eyelid and produce meibum, which is the oily substance that keeps tears from evaporating. Blinking creates negative pressure on the glands and pulls out the meibum.
The oily substance is a type of fatty acid that keeps the tear film stable. Two things can go wrong here: blockage of the release of meibum or a breakdown of the oily substance by a lipase (an enzyme that breaks down fats).[ref]
Meibomian gland blockage:
- Mites in the eyelashes can invade the meibomian glands, blocking the secretion of the meibum. (I’ll let you google for pictures of mites in your eyelashes)
- Bacterial colonization, along with dead skin cells, can also block the glands.
Breakdown of the meibum:
Phospholipases are enzymes that the body produces to break down fatty acids. The enzymes break up tear film and make the eyes dry out more quickly.
Osmolarity and dry eyes:
I mentioned above that two things go wrong with dry eyes: inflammation and increased osmolarity of the tears.
Osmolarity refers to the osmotic concentration – how much of a solute is in a solution. If we are talking about saltwater, the osmolarity would be the measure of salt particles (solute) in the water (solution).
If the water (solution) evaporates, it leads to a stronger concentration (higher osmolarity). Thus, if tears evaporate too quickly, it leads to higher osmolarity.
Tears aren’t just made up of salt and water, though, and it isn’t just a simple matter of tears being too salty. Instead, tears contain proteins, antimicrobial molecules, ions, and the aforementioned mucus and meibum.
Researchers have used several different methods to determine that sensory neurons in the eye are responsive to how concentrated the tear film is. In other words, it isn’t the amount of sodium chloride, specifically, but rather the concentration of any solute.[ref]
Sensors on the cell surface of the eyes detect osmolality. When it increases due to the evaporation of water from the tears, it triggers your eyes to blink.
Cold and Peppermint Cause My Eyes to Water:
Have you ever noticed that your eyes water when it’s cold out? Or when exposed to strong peppermint or menthol fumes? It turns out that a receptor that senses cold (and menthol) is important in regulating tear secretion. The TRPM8 receptor senses when the eye is dry (evaporation triggers the cold receptor), and it triggers blinking.
Researchers discovered that changing the osmolality (the amount of solutes, like salt) in tears also triggers a change in the TRPM8 receptor.[ref]
Inflammatory cytokines also cause changes in the sensitivity of TRPM8.[ref]
Neurogenic inflammation: long term effects
The surface of the eye faces all kinds of bacteria, viruses, and toxicants in the air. So it has to be ready to go with an immune response to keep the eye (and the body) healthy.
Like the skin, the eye’s surface also has a microbiome that is unique to you. Researchers have found that the bacterial microbiome of the eye can contain pathogenic bacteria, but that not everyone with pathogens has dry eyes or inflammation.[ref]
Chronically elevated inflammation of the eye’s surface can lead to neurogenic inflammation. The term refers to the damage to the nerve ending due to pathogens or inflammation.
Another component of neurogenic inflammation is the synthesis of neurotrophic factors that stimulate changes to the corneal nerve structure and eventually result in nerve degeneration.[ref][ref]
Think of this as a loop of localized inflammatory cytokines triggering an inflammatory response in the nerves of the eye. The other side of the loop is that triggering the nerves can cause an inflammatory response in the eye, such as in dry eyes after corneal surgery.
Neurogenic inflammation and stimulation of the trigeminal nerve are also linked to migraines. The TRMP8 receptor is also linked to migraines for some people.
Other causes of inflammation and dry eyes:
Lacrimal gland inflammation:
The lacrimal gland is where tears are formed. Inflammation here – whether from an infection or another source – can alter tears.
“An inflamed lacrimal gland may produce ‘toxic tears’ containing proinflammatory cytokines, disrupting ocular surface homeostasis and exacerbating the innate inflammatory response.”[ref]
Rosacea:
People with rosacea can get ocular rosacea, which causes red, itchy eyes or dry eyes that feel gritty. This is an autoimmune cause of dry eyes — excess inflammation due to the autoimmune condition rosacea.
IBD:
Crohn’s disease, an autoimmune condition causing inflammation in the bowel, is also linked to an increased risk of dry eye syndrome.[ref]
Vitamin A deficiency:
Insufficient vitamin A in the active form is also a cause of dry eyes. The cells covering the surface of the cornea express vitamin A receptors, and a lack of the retinol form of vitamin A has been shown to cause dry eyes due to increased inflammatory cytokines.[ref]
Symptoms of vitamin A deficiency can include poor night vision, dry eyes, and Bitot’s spots, which are white spots on the conjunctiva. (I’ll let you google Bitot’s spots for pics).
Antibiotics:
Using oral antibiotics, especially tetracycline, has been linked to an increased risk of dry eyes.[ref] The antibiotics are likely changing the microbiome of the eye surface.
Aging causes increased inflammation, driving dry eye disease
I mentioned above that I didn’t want to accept the explanation of ‘you’re getting older’ as a reason for dry eyes. But age is actually the biggest risk factor for dry eyes, with 15-30% of people over age 65 dealing with dry eyes.
As we age, we have both a decrease in the ability to fight off pathogens and an increase in chronically elevated inflammatory cytokines. As seen in autoimmune diseases, a shift towards an imbalance in the Th1 immune response may be at the root of age-related dry eyes.[ref]
A recent animal study showed an increase in antigen-presenting cells that accumulate on the eyes’ surface in aging, causing inflammation and dry eyes. Interferon-gamma activation seems to be at least a part of the problem here.[ref]
Another animal study showed that the Nrf2 pathway could be activated to decrease inflammation in the lacrimal gland found in aging.[ref]
A repeated theme in research on aging shows elevated inflammatory cytokines play a causal role in most chronic diseases associated with age. Dry eyes fit this pattern as well, and it is one more reason to target chronic inflammation as part of healthy longevity.
Dry Eyes Genotype Report:
Access this content:
An active subscription is required to access this content.
Lifehacks for dry eyes: Supplements and Lifestyle changes
Common sense: Talk with your eye doctor if you have concerns about dry eyes. These lifehacks are geared toward people who have already ruled out eye diseases and Sjogren’s syndrome (an autoimmune disease that attacks cells producing tears and saliva).
8 Lifestyle factors that may help with dry eyes:
Avoid dehydration:
Make sure you’re not dehydrated. Try drinking a glass of water first thing in the morning when you get up and then regularly drinking it throughout the day.
Increase humidity:
Low humidity exacerbates dry eye disease in a couple of ways. Decreased relative humidity causes faster evaporation, and it is also linked to an increase in HMGB1, which causes an increase in inflammation.[ref]
Air conditioning in the summer and dry heat in the winter can both reduce your indoor humidity levels significantly.
So what does your humidity need to be? An indoor humidity level of 35-45% seems to be considered normal. Studies on dry eyes show that 15 minutes in a chamber with 5% humidity causes increased blinking and dry eye symptoms.[ref]
One (free) way to increase the humidity inside your house in the winter is to hang your wet clothes on a laundry drying rack instead of using the dryer. Additionally, opening your dishwasher when it is finished washing can release moisture into the house.
Outdoor air quality index:
Exposure to higher ozone levels in the air is associated with increased dry eye symptoms and decreased tear formation.[ref] If you live in an area where air pollution is a problem, keep track of whether higher ozone days correlate to dry eyes for you.
Wind protection:
If you are outside in the sun and wind (biking, skiing, surfing, etc.), wear protective eyewear to keep air from blowing over the eyes and evaporating your tears.
Access this content:
An active subscription is required to access this content.
Related Articles and Genes:
Age-Related Macular Degeneration Genes
Age-related macular degeneration (AMD) is the most common cause of blindness in the elderly. You will find supplements specifically promoted for preventing AMD. This article explains age-related macular degeneration, delves into the genetic risks, and then explains which supplements are likely to be protective and which may do more harm than good.
Always tired? Genetic reasons for fatigue
Are you always tired even when you know you slept well? Discover more about the newest research on fatigue and how genetic susceptibility plays a part for some people.
Genetics of Double Eyelashes
Elizabeth Taylor stood out in many ways – in part because of her thick lashes. Turns out a genetic variant is the likely source of her double row of lashes. Learn more about this variant and its other associated risks.
BCO1 Gene: Converting Beta-Carotene to Vitamin A
Genetics plays a huge role in how well you convert beta-carotene into vitamin A! Discover how well you convert beta-carotene into retinol.
References:
Alam, Jehan, et al. “Retinoid Regulation of Ocular Surface Innate Inflammation.” International Journal of Molecular Sciences, vol. 22, no. 3, Feb. 2021. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms22031092.
Alven, Alyce, et al. “Impact of Low Humidity on Damage-Associated Molecular Patterns at the Ocular Surface during Dry Eye Disease.” Optometry and Vision Science: Official Publication of the American Academy of Optometry, vol. 98, no. 11, Nov. 2021, pp. 1231–38. PubMed, https://doi.org/10.1097/OPX.0000000000001802.
Askeroglu, Ufuk, et al. “Pharmaceutical and Herbal Products That May Contribute to Dry Eyes.” Plastic and Reconstructive Surgery, vol. 131, no. 1, Jan. 2013, pp. 159–67. PubMed, https://doi.org/10.1097/PRS.0b013e318272a00e.
Bian, Fang, et al. “Age-Associated Antigen-Presenting Cell Alterations Promote Dry-Eye Inducing Th1 Cells.” Mucosal Immunology, vol. 12, no. 4, July 2019, p. 897. www.ncbi.nlm.nih.gov, https://doi.org/10.1038/s41385-018-0127-z.
Contreras-Ruiz, Laura, et al. “Polymorphism in THBS1 Gene Is Associated with Post-Refractive Surgery Chronic Ocular Surface Inflammation.” Ophthalmology, vol. 121, no. 7, July 2014, pp. 1389–97. PubMed, https://doi.org/10.1016/j.ophtha.2014.01.033.
—. “Polymorphism in THBS1 Gene Is Associated with Post-Refractive Surgery Chronic Ocular Surface Inflammation.” Ophthalmology, vol. 121, no. 7, July 2014, pp. 1389–97. PubMed, https://doi.org/10.1016/j.ophtha.2014.01.033.
de Souza, Rodrigo G., et al. “Modulation of Oxidative Stress and Inflammation in the Aged Lacrimal Gland.” The American Journal of Pathology, vol. 191, no. 2, Feb. 2021, pp. 294–308. PubMed, https://doi.org/10.1016/j.ajpath.2020.10.013.
Dougherty, J. M., et al. “The Role of Tetracycline in Chronic Blepharitis. Inhibition of Lipase Production in Staphylococci.” Investigative Ophthalmology & Visual Science, vol. 32, no. 11, Oct. 1991, pp. 2970–75.
Entesarian, Miriam, et al. “FGF10 Missense Mutations in Aplasia of Lacrimal and Salivary Glands (ALSG).” European Journal of Human Genetics: EJHG, vol. 15, no. 3, Mar. 2007, pp. 379–82. PubMed, https://doi.org/10.1038/sj.ejhg.5201762.
Galvani, Giuseppe, and Georgia Fousteri. “PTPN22 and Islet-Specific Autoimmunity: What Have the Mouse Models Taught Us?” World Journal of Diabetes, vol. 8, no. 7, July 2017, pp. 330–36. PubMed Central, https://doi.org/10.4239/wjd.v8.i7.330.
Graham, Joanna E., et al. “Ocular Pathogen or Commensal: A PCR-Based Study of Surface Bacterial Flora in Normal and Dry Eyes.” Investigative Ophthalmology & Visual Science, vol. 48, no. 12, Dec. 2007, pp. 5616–23. Silverchair, https://doi.org/10.1167/iovs.07-0588.
Hallak, Joelle A., et al. “Single Nucleotide Polymorphisms in the BDNF, VDR, and DNASE 1 Genes in Dry Eye Disease Patients: A Case-Control Study.” Investigative Ophthalmology & Visual Science, vol. 56, no. 10, Sept. 2015, p. 5990. www.ncbi.nlm.nih.gov, https://doi.org/10.1167/iovs.15-17036.
—. “Single Nucleotide Polymorphisms in the BDNF, VDR, and DNASE 1 Genes in Dry Eye Disease Patients: A Case-Control Study.” Investigative Ophthalmology & Visual Science, vol. 56, no. 10, Sept. 2015, p. 5990. www.ncbi.nlm.nih.gov, https://doi.org/10.1167/iovs.15-17036.
Hwang, Daniel Duck-Jin, et al. “The Role of Neuropeptides in Pathogenesis of Dry Dye.” Journal of Clinical Medicine, vol. 10, no. 18, Sept. 2021. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/jcm10184248.
Imbert, Yoannis, et al. “MUC1 and Estrogen Receptor Alpha Gene Polymorphisms in Dry Eye Patients.” Experimental Eye Research, vol. 88, no. 3, Mar. 2009, pp. 334–38. PubMed, https://doi.org/10.1016/j.exer.2008.05.019.
Lam, Sin Man, et al. “Longitudinal Changes in Tear Fluid Lipidome Brought about by Eyelid-Warming Treatment in a Cohort of Meibomian Gland Dysfunction.” Journal of Lipid Research, vol. 55, no. 9, Sept. 2014, p. 1959. www.ncbi.nlm.nih.gov, https://doi.org/10.1194/jlr.P051185.
Ling, Yu-Hsiang, et al. “TRPM8 Genetic Variant Is Associated with Chronic Migraine and Allodynia.” The Journal of Headache and Pain, vol. 20, no. 1, Dec. 2019, p. 115. PubMed, https://doi.org/10.1186/s10194-019-1064-2.
Liu, Yi, et al. “HMBG1 as a Driver of Inflammatory and Immune Processes in the Pathogenesis of Ocular Diseases.” Journal of Ophthalmology, vol. 2018, Oct. 2018, p. e5195290. www.hindawi.com, https://doi.org/10.1155/2018/5195290.
Matossian, Cynthia, et al. “Dry Eye Disease: Consideration for Women’s Health.” Journal of Women’s Health, vol. 28, no. 4, Apr. 2019, p. 502. www.ncbi.nlm.nih.gov, https://doi.org/10.1089/jwh.2018.7041.
Meng, Yi-Fang, et al. “Association Between Single Nucleotide Polymorphisms in the Vitamin D Receptor and Incidence of Dry Eye Disease in Chinese Han Population.” Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, vol. 25, 2019, p. 4759. www.ncbi.nlm.nih.gov, https://doi.org/10.12659/MSM.915434.
Na, Kyung-Sun, et al. “Proinflammatory Gene Polymorphisms Are Potentially Associated with Korean Non-Sjogren Dry Eye Patients.” Molecular Vision, vol. 17, 2011, p. 2818. www.ncbi.nlm.nih.gov, https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC3224841/.
Ozan, Erol, et al. “The Effect of Depression, BDNF Gene Val66met Polymorphism and Gender on Serum BDNF Levels.” Brain Research Bulletin, vol. 81, no. 1, Jan. 2010, pp. 61–65. ScienceDirect, https://doi.org/10.1016/j.brainresbull.2009.06.022.
Quallo, Talisia, et al. “TRPM8 Is a Neuronal Osmosensor That Regulates Eye Blinking in Mice.” Nature Communications, vol. 6, 2015. www.ncbi.nlm.nih.gov, https://doi.org/10.1038/ncomms8150.
Rodriguez-Rodriguez, Luis, et al. “The Rs3771863 Single Nucleotide Polymorphism of the TACR1 Gene Is Associated to a Lower Risk of Sicca Syndrome in Fibromyalgia Patients.” Clinical and Experimental Rheumatology, vol. 33, no. 1 Suppl 88, Feb. 2015, pp. S33-40.
Safonova, T. N., G. V. Zaitseva, et al. “[Association of polymorphic markers rs7947461 of the TRIM21 gene and rs33996649 of the PTPN22 gene with the risk of developing exogenous dry eye syndrome].” Vestnik Oftalmologii, vol. 137, no. 5. Vyp. 2, 2021, pp. 217–23. PubMed, https://doi.org/10.17116/oftalma2021137052217.
Safonova, T. N., Z. V. Surnina, et al. “The Role of Polymorphic Markers Rs1478604, Rs2292305, and Rs2228262 in THBS1 Gene in the Development of Autoimmune Dry Eye Syndrome.” Bulletin of Experimental Biology and Medicine, vol. 169, no. 5, Sept. 2020, pp. 707–09. PubMed, https://doi.org/10.1007/s10517-020-04960-0.
Stern, Michael E., et al. “Conjunctival T-Cell Subpopulations in Sjögren’s and Non-Sjögren’s Patients with Dry Eye.” Investigative Ophthalmology & Visual Science, vol. 43, no. 8, Aug. 2002, pp. 2609–14.
Tsubota, Kazuo, et al. “Defining Dry Eye from a Clinical Perspective.” International Journal of Molecular Sciences, vol. 21, no. 23, Dec. 2020. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms21239271.
—. “Defining Dry Eye from a Clinical Perspective.” International Journal of Molecular Sciences, vol. 21, no. 23, Dec. 2020. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms21239271.
—. “Defining Dry Eye from a Clinical Perspective.” International Journal of Molecular Sciences, vol. 21, no. 23, Dec. 2020. www.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms21239271.
Yang, Shen, et al. “PTPN22 Phosphorylation Acts as a Molecular Rheostat for the Inhibition of TCR Signaling.” Science Signaling, vol. 13, no. 623, Mar. 2020, p. eaaw8130. PubMed, https://doi.org/10.1126/scisignal.aaw8130.
Zhebrun, Daria, et al. “Association of PTPN22 1858T/T Genotype with Type 1 Diabetes, Graves’ Disease but Not with Rheumatoid Arthritis in Russian Population.” Aging (Albany NY), vol. 3, no. 4, Apr. 2011, pp. 368–73. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3117451/.