Key takeaways:
~ Estrogen is a hormone that turns on or off the transcription of estrogen-responsive genes, many of which are involved in cell growth.
~ The synthesis of estrogen and the breakdown of estrogen are tightly regulated.
~ Genetic variants in the estrogen metabolism pathways impact the risk of breast or prostate cancer.
~ Environmental estrogen mimics, such as those from plastics, can also interact with the way your body metabolizes and balances estrogen levels.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
Estrogen creation and metabolism
Estrogen is a steroid hormone that is synthesized from cholesterol and is important in reproduction, bone density, heart health, and brain health.
There are several different types of estrogen, and it is a hormone important in both males and females, although at different levels. Estrogen – from how much is made to how it is broken down – is dependent on both genetics and lifestyle factors affecting both men and women.
Let’s get started by looking at the types of estrogen and how it is created, including the genes involved. Then we will dive into the research on estrogen metabolites and how they can affect your health. Genetic variants in the estrogen metabolism genes can affect cancer risk and your body’s tolerance for estrogen-mimicking chemicals.
Types of estrogen:
There are several forms of estrogen in the body, and the amounts of each type become important for hormone-related cancer risk and uterine fibroids.[ref]
- Estradiol (E2) or 17β-Estradiol – the primary form in women before menopause
- Estrone (E1) -primarily made after menopause, the primary form in men
- Estriol (E3) – the main type of estrogen during pregnancy
- Estretrol (E4) – only during pregnancy, made by the fetus
For the most part, we will focus here on estradiol (E2) and estriol. In addition to these types of estrogen, there are also estrogen metabolites created from the breakdown of estrogen, which we will cover below.
How is estrogen created in the body?
Estrogen is synthesized in the following tissues:[ref]
- ovaries (major source in women, E2)
- testes (males)
- fat cells (E1, especially post-menopause)
- brain
- liver
- pancreas
- intestines
- adrenals
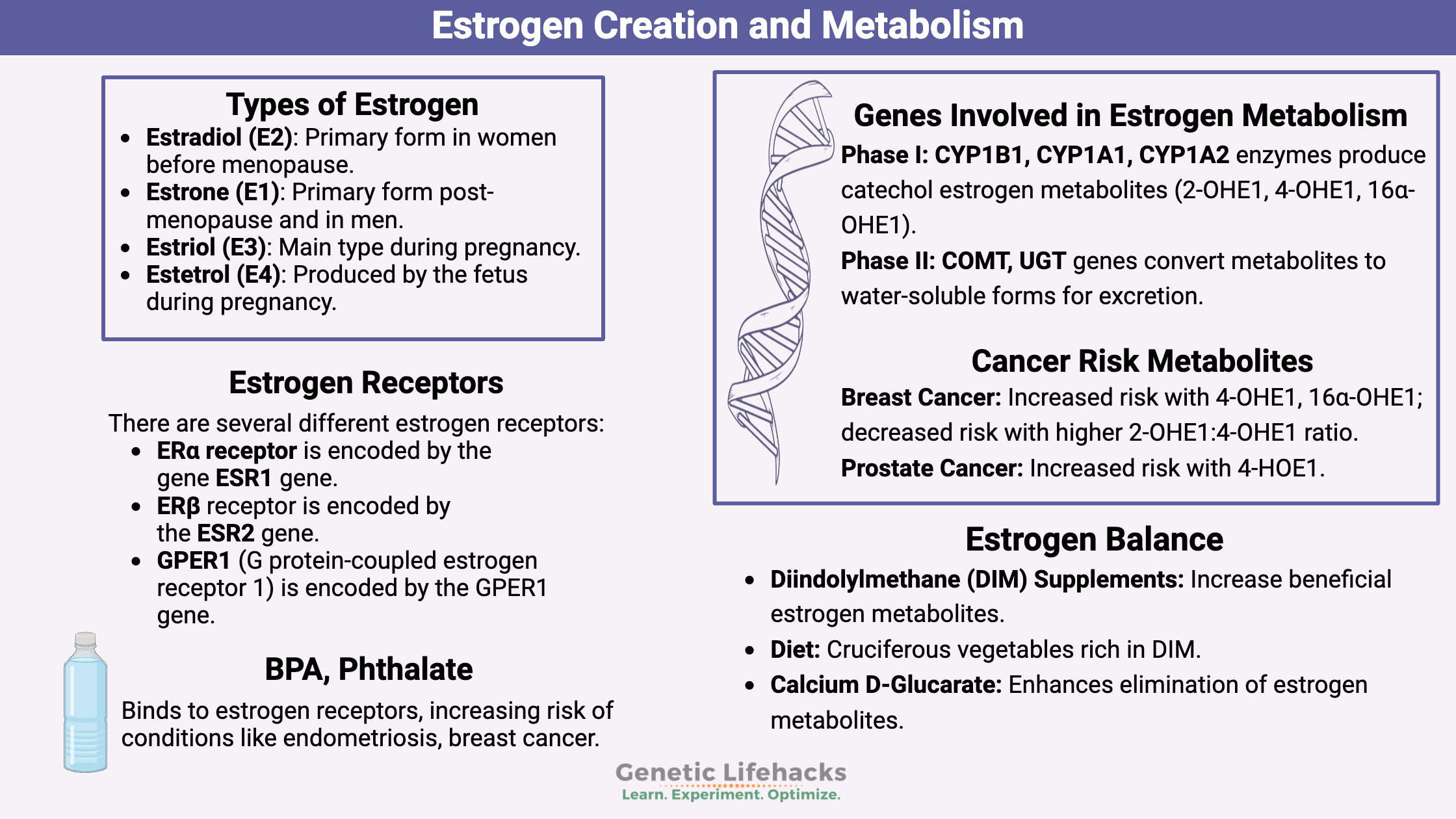
The precursor for estrogen is cholesterol, which is first converted (using CYP11A1) into progesterone, which is then converted (using CYP17A1) into androstenedione.
Androstenedione can be converted into testosterone, dihydrotestosterone, or estrogen.
If it goes the estrogen route, androstenedione is converted (using CYP19A1) first to estrone (E1), which is then converted (using 17β-HSD) into estradiol.[ref]
Here is a flow chart to give you a better idea of the conversion of cholesterol into estrogen, including the genes that are involved.
Estrogen Sulfate: Storage form of estrogen
On a hormone lab panel test, you will likely see estrogen sulfate listed. Estrogen sulfate is the most abundant form of estrogen, but it is also not very active. It can be considered as a storage form of estrogen that can be converted by HSD17B1 (17β-Hydroxysteroid dehydrogenase) into estradiol. High levels of estrogen sulfate can be a risk factor for breast cancer.
Driving estrogen production: FSH
Within follicle cells in the ovary, the conversion of the steroid hormone precursor into estrogen is controlled by follicle-stimulating hormone (FSH) levels. FSH is produced in the pituitary gland, and, along with luteinizing hormone (LH), controls the menstrual cycle.
Estrogen Receptors: Controlling Genes
So what exactly does estrogen do in cells?
Estrogen is transported throughout the body and can bind to estrogen receptors in the cell nucleus, controlling the transcription of many different genes. Thus, the different estrogen receptors can control whether a gene gets transcribed into a protein that is used in the cell.
There are several different estrogen receptors:
- ERα receptor is encoded by the ESR1 gene.
- ERβ receptor is encoded by the ESR2 gene.
- GPER1 (G protein-coupled estrogen receptor 1) is encoded by the GPER1 gene.
The estrogen receptors can bind to and turn on hundreds of different genes. Some important targets of estrogen include the LDL receptor, progesterone receptor, IGF-1, and many more. These genes are related to hormones, cholesterol, and growth within the body.[ref]
Estrogen receptors (ERs) are present in a wide variety of tissues in the body. For example, ERs are important in vascular endothelial cells, which line blood vessels. Estrogen receptors are found in cardiomyocytes (heart muscle cells), neurons, airway cells, muscles, the uterus, testes, fat tissue, bone, breast, kidneys, and more.
Estrogen receptors can also cause rapid activation of signaling pathways or immediate changes in certain cells. Estrogen can also impact mitochondrial biogenesis, function, and regulation of ATP production.[ref]
In a nutshell, estrogen causes the body to increase the production of other hormones, growth factors, and metabolic factors.
What does estrogen do in the body?
For women, estrogen regulates the menstrual cycle and is imperative for reproduction. The primary and secondary sexual characteristics of women (breasts, wider hips, lack of facial hair, etc) are due to estrogen production starting in puberty.
For men and women, estrogen is also important in maintaining bone density and cognitive function. Low estrogen is linked to osteoporosis. It is also important in brain function and controlling inflammation.[ref]
In men, estrogen is also necessary at low levels in the production of sperm. The loss of the estrogen receptor in the testes results in abnormal sperm. On the other hand, too much estrogen can also be detrimental to male reproductive health.[ref] Balance is key.
What happens when you have too much estrogen?
Signs of excess estrogen in women include:
- weight gain
- heavy periods
- fibroids
- PMS
- fibrocystic breasts
- loss of sex drive
- fatigue, depression, anxiety
For men, too much estrogen leads to:
- gynecomastia
- sexual dysfunction
- loss of muscle mass
- fatigue, depression, anxiety
Outside of estrogen’s essential role in reproduction, estrogen plays a key role in the control of energy metabolism.[ref]
Getting rid of estrogen (metabolism or breakdown and elimination):
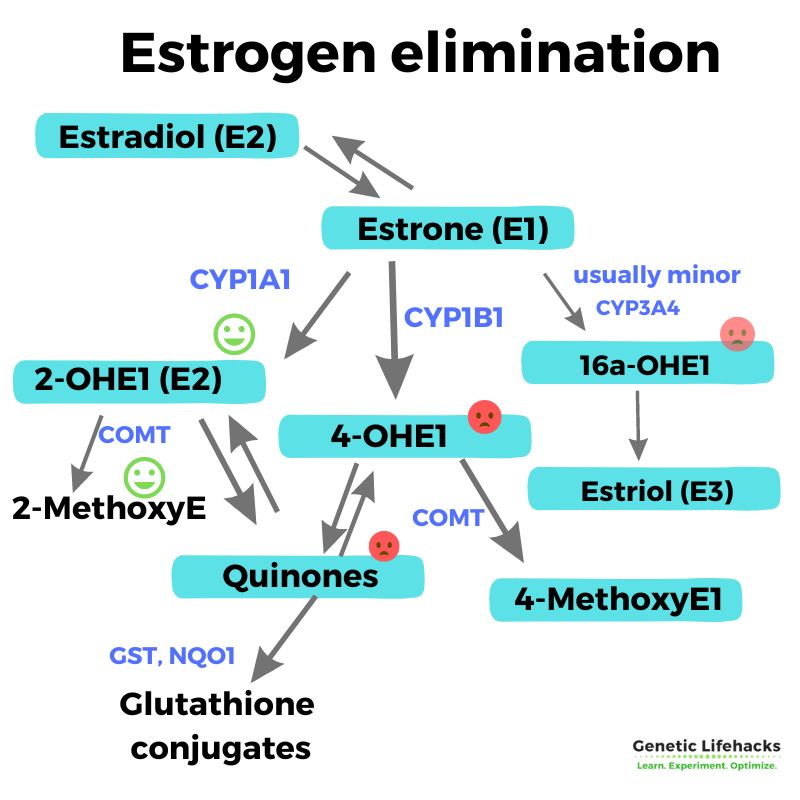
The level of estrogen in the body needs to be at the right level for the individual’s age and sex. To control the level of estrogen in the body, we have to have multiple ways to break it down and eliminate it. This is a multi-step process involving what are known as phase I and phase II detoxification enzymes.
Phase I and Phase II estrogen metabolism:
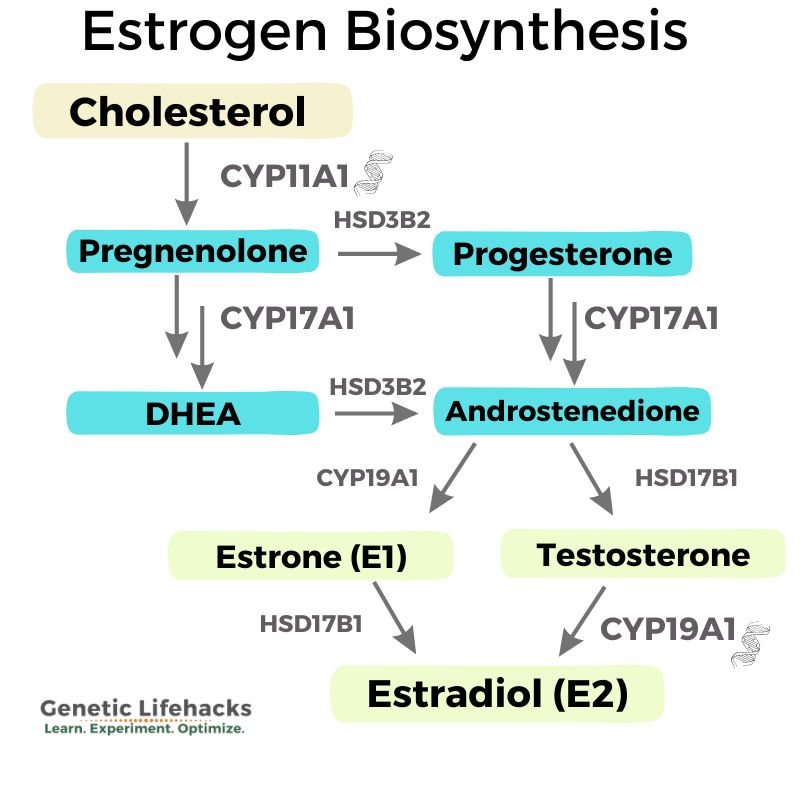
In the liver, the CYP450 enzymes can metabolize estrogen. Specifically, this is done by the CYP1B1, CYP1A1, or CYP1A2 enzymes.
This process creates metabolites known as 2-OHE1 (E2), 4-OHE1(E2), and 16α-OHE1, all of which are also known as catechol estrogen metabolites.
These catechol estrogen metabolites can be further changed by the COMT (catechol-O-methyltransferase) enzyme or through glucuronidation (UGT genes). This makes them water-soluble and able to be excreted through urine or feces.[ref]
Essentially, this two-step process needs to work in tandem:
- Phase I: The CYP1B1 or CYP1A1 enzyme breaks down estradiol into the catechol estrogen metabolites.
- Phase II: The metabolites are converted into water-soluble substances (by COMT, UGTs) for elimination.
It is important that the two phases of estrogen metabolism act in sync.
Some of the metabolites, such as 16α-OHE1, are also able to activate the estrogen receptors. These specific estrogen metabolites increase the risk of breast cancer.[ref] Thus, you don’t want certain Phase I metabolites hanging around in the body.
Estrogen metabolites linked to breast, ovarian, and prostate cancer:
For breast cancer, the 4-OHE1(E2) and 16α-OHE1 metabolites are implicated in increasing the relative risk of cancer.
Higher amounts of 2-OHE1(E2) or a better ratio of 2-OHE1:4-OHE1 decrease breast cancer risk. Additionally, you don’t want too much estrogen (E1 or E2), in general, hanging around. Everything needs to be in balance.[ref][ref]
Prostate cancer risk is increased with 4-HOE1(E2) metabolites also.[ref]
In general, the estrogen metabolites that start with “2” are good, and the ones that start with “4” or “16” need to be limited or at least eliminated quickly from the body.[ref]
Here is a diagram of how these metabolites go together:
As you can see, upregulating the CYP1A1 enzyme is going to increase the 2-OHE1 path.
Too much estrogen being metabolized through CYP1B1 into 4-OHE1(E2) and the estrogen quinones can potentially be bad if your body has slower phase II (COMT, GSTP1, GSTM1, NQO1) enzymes.[ref][ref]
The connection between smoking and estrogen-related cancers:
Smoking significantly increases the risk of breast cancer and prostate cancer.
Part of the reason smoking increases cancer risk in general is that it can cause DNA damage. However, smoking specifically increases the risk of breast cancer through upregulating the CYP1B1 and CYP1A1 enzymes. For people with genetic variants that cause more of an impact on CYP1B1 upregulation – combined with an inability to eliminate the estrogen metabolites fast enough (due to phase II genes, diet, and lifestyle) – then cigarette smoking is going to significantly increase the ‘bad’ estrogen metabolites. Smoking also may impair the phase II metabolites, thus creating more estrogen quinone metabolites with a decreased ability to eliminate them.[ref][ref]
Therefore, combining some of phase I and phase II genetic variants (see the genotype report below) with smoking causes a fairly large increase in the risk of cervical, breast, or prostate cancer.
Estrogen Elimination: Phase III
Let’s go one step further and make the two-step process of estrogen metabolism into a three-step process…
Once the catechol estrogen metabolites have been metabolized (COMT), they have to be excreted (urine or feces).
The gut microbiome comes into play here in making sure that the metabolites are excreted and not reabsorbed. The estrogen that has been metabolized and is ready to be eliminated through feces can actually be recycled back into circulation due to an interaction with certain bacteria in your gut microbiome.
Beta-glucuronidase, an enzyme produced by the gut microbiome, can reverse the reaction that the UGT enzymes did to make the estrogen metabolites more water-soluble. This can cause the estrogen metabolites to be reabsorbed from the intestines and go back into circulation.[ref]
Calcium D-glucarate can suppress the beta-glucuronidase activity in the gut, thus increasing the number of estrogen metabolites that are excreted.[ref]
Estrogen Mimics: BPA, Phthalates, and Other Toxicants
There are several environmental toxicants that act similarly to estrogen in the body. Among these, phthalates and BPA are ubiquitous, with research showing that almost everyone has them in their bodies. These estrogen-mimicking chemicals can bind to the estrogen receptors, similarly to the way that estrogen binds.[ref][ref]
Phthalates are used in vinyl, plastics, adhesives, artificial fragrances (laundry detergent, air freshener), personal care products, and more.[ref]
Related article: Phthalates and detoxification pathways
BPA is also found in plastics, and we are exposed through food and drinks being stored in plastic containers or cans with linings containing BPA. Even the paperboard used in food packaging (especially if it is recycled cardboard) can contain BPA, which is then transferred to the food we eat.[ref]
Related article: BPA, BPS and detoxification pathways
These estrogen mimics (at the levels found in people every day) have been linked to increased risk of several estrogen-related conditions, including:
- endometriosis[ref]
- enlarged prostate[ref]
- almost 2-fold increase in breast cancer for higher phthalate exposure (estrogen receptor-positive)[ref]
- BPA exposure at low levels is linked to increased breast and prostate cancer[ref]
- uterine fibroids[ref][ref]
- platelet reactivity and heart disease[ref][ref]
We will cover more on how to avoid and eliminate these estrogen mimics in the Lifehacks section. First, though, let’s cover how your genes interact with estrogen metabolism (phase I and phase II) and how your genes impact estrogen creation.
Estrogen Genotype Report:
This section shows how your genes make you unique when it comes to estrogen metabolism and estrogen synthesis. Most are common genetic variants, and almost everyone will have a few of the variants. Do not be overly worried if you have a variant that increases the relative risk of breast or prostate cancer. The goal is to use the information to make changes – dietary and lifestyle – to minimize your risk factors.
Member Content:
Not a member?
Lifehacks for estrogen balance:
Testing is the only way to truly know your estrogen levels. You can order your own hormone test panels online (e.g. UltaLabs Estrogen Panel) or go through your doctor. A qualified professional can help you make sense of your hormone test results.
The lifehacks below are based on natural supplements and lifestyle changes for reducing estrogen and shifting towards the less-risky estrogen metabolites. Please also talk with your doctor about prescription-based options and be sure to discuss any supplement interactions if you are on medications.
Optimizing Phase I detoxification for estrogen metabolism:
Member Content:
Not a member?
References:
Abbas, Mohammad, et al. “Association of CYP1A1 Gene Variants Rs4646903 (T>C) and Rs1048943 (A>G) with Cervical Cancer in a North Indian Population.” European Journal of Obstetrics, Gynecology, and Reproductive Biology, vol. 176, May 2014, pp. 68–74. PubMed, doi:10.1016/j.ejogrb.2014.02.036.Ahern, Thomas P., et al. “Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study.” Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, vol. 37, no. 21, July 2019, pp. 1800–09. PubMed, doi:10.1200/JCO.18.02202.Barakat, Radwa, et al. “Extra-Gonadal Sites of Estrogen Biosynthesis and Function.” BMB Reports, vol. 49, no. 9, Sept. 2016, pp. 488–96. PubMed Central, doi:10.5483/BMBRep.2016.49.9.141.Bayer, Janine, et al. “Estrogen and the Male Hippocampus: Genetic Variation in the Aromatase Gene Predicting Serum Estrogen Is Associated with Hippocampal Gray Matter Volume in Men.” Hippocampus, vol. 23, no. 2, Feb. 2013, pp. 117–21. PubMed, doi:10.1002/hipo.22059.Bekö, Gabriel, et al. “Children’s Phthalate Intakes and Resultant Cumulative Exposures Estimated from Urine Compared with Estimates from Dust Ingestion, Inhalation and Dermal Absorption in Their Homes and Daycare Centers.” PLoS ONE, vol. 8, no. 4, Apr. 2013. PubMed Central, doi:10.1371/journal.pone.0062442.Beuten, Joke, et al. “CYP1B1 Variants Are Associated with Prostate Cancer in Non-Hispanic and Hispanic Caucasians.” Carcinogenesis, vol. 29, no. 9, Sept. 2008, pp. 1751–57. PubMed, doi:10.1093/carcin/bgm300.Butts, Samantha F., et al. “Joint Effects of Smoking and Gene Variants Involved in Sex Steroid Metabolism on Hot Flashes in Late Reproductive-Age Women.” The Journal of Clinical Endocrinology and Metabolism, vol. 97, no. 6, June 2012, pp. E1032–42. PubMed Central, doi:10.1210/jc.2011-2216.—. “Joint Effects of Smoking and Gene Variants Involved in Sex Steroid Metabolism on Hot Flashes in Late Reproductive-Age Women.” The Journal of Clinical Endocrinology and Metabolism, vol. 97, no. 6, June 2012, pp. E1032–42. PubMed Central, doi:10.1210/jc.2011-2216.Cao, X. L., et al. “Concentrations of Bisphenol A in the Composite Food Samples from the 2008 Canadian Total Diet Study in Quebec City and Dietary Intake Estimates.” Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, vol. 28, no. 6, June 2011, pp. 791–98. PubMed Central, doi:10.1080/19440049.2010.513015.Cavalieri, Ercole L., and Eleanor G. Rogan. “Etiology and Prevention of Prevalent Types of Cancer.” Journal of Rare Diseases Research & Treatment, vol. 2, no. 3, 2017, pp. 22–29. PubMed Central, doi:10.29245/2572-9411/2017/3.1093.Cerne, Jasmina-Ziva, et al. “Combined Effect of CYP1B1, COMT, GSTP1, and MnSOD Genotypes and Risk of Postmenopausal Breast Cancer.” Journal of Gynecologic Oncology, vol. 22, no. 2, June 2011, pp. 110–19. PubMed, doi:10.3802/jgo.2011.22.2.110.Chang, Wei-Hsiang, et al. “Sex Hormones and Oxidative Stress Mediated Phthalate-Induced Effects in Prostatic Enlargement.” Environment International, vol. 126, 2019, pp. 184–92. PubMed, doi:10.1016/j.envint.2019.02.006.Chaudhary, Amit, and Kristine L. Willett. “Inhibition of Human Cytochrome CYP 1 Enzymes by Flavonoids of St. John’s Wort.” Toxicology, vol. 217, no. 2–3, Jan. 2006, pp. 194–205. PubMed, doi:10.1016/j.tox.2005.09.010.Chen, Z. P., et al. “The Single Nucleotide Polymorphism Rs700518 Is an Independent Risk Factor for Metabolic Syndrome and Benign Prostatic Hyperplasia (MetS‐BPH).” Andrology, vol. 6, no. 4, July 2018, pp. 568–78. PubMed Central, doi:10.1111/andr.12498.
Cooke, Paul S., et al. “Estrogens in Male Physiology.” Physiological Reviews, vol. 97, no. 3, July 2017, pp. 995–1043. PubMed Central, doi:10.1152/physrev.00018.2016.
—. “Estrogens in Male Physiology.” Physiological Reviews, vol. 97, no. 3, July 2017, pp. 995–1043. PubMed Central, doi:10.1152/physrev.00018.2016.
Cote, Michele L., et al. “Tobacco and Estrogen Metabolic Polymorphisms and Risk of Non-Small Cell Lung Cancer in Women.” Carcinogenesis, vol. 30, no. 4, Apr. 2009, pp. 626–35. PubMed Central, doi:10.1093/carcin/bgp033.
Draz, Hossam, et al. “Diindolylmethane and Its Halogenated Derivatives Induce Protective Autophagy in Human Prostate Cancer Cells via Induction of the Oncogenic Protein AEG-1 and Activation of AMP-Activated Protein Kinase (AMPK).” Cellular Signalling, vol. 40, 2017, pp. 172–82. PubMed, doi:10.1016/j.cellsig.2017.09.006.
—. “Diindolylmethane and Its Halogenated Derivatives Induce Protective Autophagy in Human Prostate Cancer Cells via Induction of the Oncogenic Protein AEG-1 and Activation of AMP-Activated Protein Kinase (AMPK).” Cellular Signalling, vol. 40, 2017, pp. 172–82. PubMed, doi:10.1016/j.cellsig.2017.09.006.
Dvorakova, Marketa, et al. “Selected Bisphenols and Phthalates Screened for Estrogen and Androgen Disruption by in Silico and in Vitro Methods.” Neuro Endocrinology Letters, vol. 39, no. 5, Dec. 2018, pp. 409–16.
Dwivedi, C., et al. “Effect of Calcium Glucarate on Beta-Glucuronidase Activity and Glucarate Content of Certain Vegetables and Fruits.” Biochemical Medicine and Metabolic Biology, vol. 43, no. 2, Apr. 1990, pp. 83–92.
Fuentes, Nathalie, and Patricia Silveyra. “Estrogen Receptor Signaling Mechanisms.” Advances in Protein Chemistry and Structural Biology, vol. 116, 2019, pp. 135–70. PubMed Central, doi:10.1016/bs.apcsb.2019.01.001.
—. “Estrogen Receptor Signaling Mechanisms.” Advances in Protein Chemistry and Structural Biology, vol. 116, 2019, pp. 135–70. PubMed Central, doi:10.1016/bs.apcsb.2019.01.001.
—. “Estrogen Receptor Signaling Mechanisms.” Advances in Protein Chemistry and Structural Biology, vol. 116, 2019, pp. 135–70. PubMed Central, doi:10.1016/bs.apcsb.2019.01.001.
—. “Estrogen Receptor Signaling Mechanisms.” Advances in Protein Chemistry and Structural Biology, vol. 116, 2019, pp. 135–70. PubMed Central, doi:10.1016/bs.apcsb.2019.01.001.
Giudice, Aldo, et al. “Dissecting the Prevention of Estrogen-Dependent Breast Carcinogenesis through Nrf2-Dependent and Independent Mechanisms.” OncoTargets and Therapy, vol. 12, June 2019, pp. 4937–53. PubMed Central, doi:10.2147/OTT.S183192.
—. “Dissecting the Prevention of Estrogen-Dependent Breast Carcinogenesis through Nrf2-Dependent and Independent Mechanisms.” OncoTargets and Therapy, vol. 12, June 2019, pp. 4937–53. PubMed Central, doi:10.2147/OTT.S183192.
Goodman, M. T., et al. “Case-Control Study of Ovarian Cancer and Polymorphisms in Genes Involved in Catecholestrogen Formation and Metabolism.” Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, vol. 10, no. 3, Mar. 2001, pp. 209–16.
Gu, Cheng-Yuan, et al. “A Single Nucleotide Polymorphism in CYP1B1 Leads to Differential Prostate Cancer Risk and Telomere Length.” Journal of Cancer, vol. 9, no. 2, Jan. 2018, pp. 269–74. PubMed Central, doi:10.7150/jca.21774.
Gurley, Bill J., et al. “Clinical Assessment of Effects of Botanical Supplementation on Cytochrome P450 Phenotypes in the Elderly: St John’s Wort, Garlic Oil, Panax Ginseng and Ginkgo Biloba.” Drugs & Aging, vol. 22, no. 6, 2005, pp. 525–39. PubMed, doi:10.2165/00002512-200522060-00006.
Hehn, Rebecca Simonne. “NHANES Data Support Link between Handling of Thermal Paper Receipts and Increased Urinary Bisphenol A Excretion.” Environmental Science & Technology, vol. 50, no. 1, Jan. 2016, pp. 397–404. PubMed, doi:10.1021/acs.est.5b04059.
Hodges, Romilly E., and Deanna M. Minich. “Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application.” Journal of Nutrition and Metabolism, vol. 2015, 2015. PubMed Central, doi:10.1155/2015/760689.
Huang, Po-Chin, et al. “Characterization of Phthalates Exposure and Risk for Cosmetics and Perfume Sales Clerks.” Environmental Pollution (Barking, Essex: 1987), vol. 233, Feb. 2018, pp. 577–87. PubMed, doi:10.1016/j.envpol.2017.10.079.
Jaeger, Cassie, and Shelley A. Tischkau. “Role of Aryl Hydrocarbon Receptor in Circadian Clock Disruption and Metabolic Dysfunction.” Environmental Health Insights, vol. 10, Aug. 2016, pp. 133–41. PubMed Central, doi:10.4137/EHI.S38343.
Jain, Vijaylakshmi, et al. “Polymorphism of CYP1A1 Gene Variants Rs4646903 and Rs1048943 Relation to the Incidence of Cervical Cancer in Chhattisgarh.” Environmental Toxicology and Pharmacology, vol. 52, June 2017, pp. 188–92. ScienceDirect, doi:10.1016/j.etap.2017.04.009.
Jaramillo-Rangel, G., et al. “Polymorphisms in GSTM1, GSTT1, GSTP1, and GSTM3 Genes and Breast Cancer Risk in Northeastern Mexico.” Genetics and Molecular Research: GMR, vol. 14, no. 2, June 2015, pp. 6465–71. PubMed, doi:10.4238/2015.June.11.22.
—. “Polymorphisms in GSTM1, GSTT1, GSTP1, and GSTM3 Genes and Breast Cancer Risk in Northeastern Mexico.” Genetics and Molecular Research: GMR, vol. 14, no. 2, June 2015, pp. 6465–71. PubMed, doi:10.4238/2015.June.11.22.
Johansson, Harriet, et al. “Prognostic Impact of Genetic Variants of CYP19A1 and UGT2B17 in a Randomized Trial for Endocrine-Responsive Postmenopausal Breast Cancer.” The Pharmacogenomics Journal, Apr. 2019. PubMed, doi:10.1038/s41397-019-0087-z.
Justenhoven, Christina. “Polymorphisms of Phase I and Phase II Enzymes and Breast Cancer Risk.” Frontiers in Genetics, vol. 3, Nov. 2012. PubMed Central, doi:10.3389/fgene.2012.00258.
Kim, E. H., et al. “A Prospective Study of Grapefruit and Grapefruit Juice Intake and Breast Cancer Risk.” British Journal of Cancer, vol. 98, no. 1, Jan. 2008, pp. 240–41. PubMed Central, doi:10.1038/sj.bjc.6604105.
Kispert, Shannon, and Jane McHowat. “Recent Insights into Cigarette Smoking as a Lifestyle Risk Factor for Breast Cancer.” Breast Cancer : Targets and Therapy, vol. 9, Mar. 2017, pp. 127–32. PubMed Central, doi:10.2147/BCTT.S129746.
Kisselev, Pyotr, et al. “Association of CYP1A1 Polymorphisms with Differential Metabolic Activation of 17β-Estradiol and Estrone.” Cancer Research, vol. 65, no. 7, Apr. 2005, pp. 2972–78. cancerres.aacrjournals.org, doi:10.1158/0008-5472.CAN-04-3543.
Ko, Jeong-Hyeon, et al. “Pharmacological Utilization of Bergamottin, Derived from Grapefruits, in Cancer Prevention and Therapy.” International Journal of Molecular Sciences, vol. 19, no. 12, Dec. 2018. PubMed Central, doi:10.3390/ijms19124048.
Lajin, B., and A. Alachkar. “The NQO1 Polymorphism C609T (Pro187Ser) and Cancer Susceptibility: A Comprehensive Meta-Analysis.” British Journal of Cancer, vol. 109, no. 5, Sept. 2013, pp. 1325–37. PubMed Central, doi:10.1038/bjc.2013.357.
Lee, Sang-Ah, et al. “Cruciferous Vegetables, the GSTP1 Ile105Val Genetic Polymorphism, and Breast Cancer Risk.” The American Journal of Clinical Nutrition, vol. 87, no. 3, Mar. 2008, pp. 753–60. PubMed, doi:10.1093/ajcn/87.3.753.
Li, Hai-Ling, et al. “Phthalates in Infant Cotton Clothing: Occurrence and Implications for Human Exposure.” The Science of the Total Environment, vol. 683, Sept. 2019, pp. 109–15. PubMed, doi:10.1016/j.scitotenv.2019.05.132.
Li, Yiwei, and Fazlul H. Sarkar. “Role of BioResponse 3,3′-Diindolylmethane in the Treatment of Human Prostate Cancer: Clinical Experience.” Medical Principles and Practice, vol. 25, no. Suppl 2, July 2016, pp. 11–17. PubMed Central, doi:10.1159/000439307.
Mandal, Raju K., et al. “Genetic Variants in Metabolizing Genes NQO1, NQO2, MTHFR and Risk of Prostate Cancer: A Study from North India.” Molecular Biology Reports, vol. 39, no. 12, Dec. 2012, pp. 11145–52. PubMed, doi:10.1007/s11033-012-2023-z.
Martínez-Ramírez, O. C., et al. “Polymorphisms of Catechol Estrogens Metabolism Pathway Genes and Breast Cancer Risk in Mexican Women.” Breast (Edinburgh, Scotland), vol. 22, no. 3, June 2013, pp. 335–43. PubMed, doi:10.1016/j.breast.2012.08.004.
Matthews, J. B., et al. “In Vitro and in Vivo Interactions of Bisphenol A and Its Metabolite, Bisphenol A Glucuronide, with Estrogen Receptors Alpha and Beta.” Chemical Research in Toxicology, vol. 14, no. 2, Feb. 2001, pp. 149–57. PubMed, doi:10.1021/tx0001833.
Monroe, K. R., et al. “Prospective Study of Grapefruit Intake and Risk of Breast Cancer in Postmenopausal Women: The Multiethnic Cohort Study.” British Journal of Cancer, vol. 97, no. 3, Aug. 2007, pp. 440–45. PubMed, doi:10.1038/sj.bjc.6603880.
Moore, Steven C., et al. “Endogenous Estrogens, Estrogen Metabolites, and Breast Cancer Risk in Postmenopausal Chinese Women.” Journal of the National Cancer Institute, vol. 108, no. 10, 2016. PubMed, doi:10.1093/jnci/djw103.
Moreira Fernandez, Miriany Avelino, et al. “Study of Possible Association between Endometriosis and Phthalate and Bisphenol A by Biomarkers Analysis.” Journal of Pharmaceutical and Biomedical Analysis, vol. 172, Aug. 2019, pp. 238–42. PubMed, doi:10.1016/j.jpba.2019.04.048.
Morgan, Marsha K., et al. “Distribution, Variability, and Predictors of Urinary Bisphenol A Levels in 50 North Carolina Adults over a Six-Week Monitoring Period.” Environment International, vol. 112, Mar. 2018, pp. 85–99. PubMed Central, doi:10.1016/j.envint.2017.12.014.
Nock, Nora L., et al. “Associations between Smoking, Polymorphisms in Polycyclic Aromatic Hydrocarbon (PAH) Metabolism and Conjugation Genes and PAH-DNA Adducts in Prostate Tumors Differ by Race.” Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, vol. 16, no. 6, June 2007, pp. 1236–45. PubMed, doi:10.1158/1055-9965.EPI-06-0736.
Oussalah, Abderrahim, et al. “Exome-Wide Association Study Identifies New Low-Frequency and Rare UGT1A1 Coding Variants and UGT1A6 Coding Variants Influencing Serum Bilirubin in Elderly Subjects.” Medicine, vol. 94, no. 22, June 2015. PubMed Central, doi:10.1097/MD.0000000000000925.
Pearce, C. L., et al. “Validating Genetic Risk Associations for Ovarian Cancer through the International Ovarian Cancer Association Consortium.” British Journal of Cancer, vol. 100, no. 2, Jan. 2009, pp. 412–20. PubMed Central, doi:10.1038/sj.bjc.6604820.
Peng, Qiliu, et al. “The NQO1 Pro187Ser Polymorphism and Breast Cancer Susceptibility: Evidence from an Updated Meta-Analysis.” Diagnostic Pathology, vol. 9, May 2014, p. 100. PubMed Central, doi:10.1186/1746-1596-9-100.
“PharmGKB.” PharmGKB, https://www.pharmgkb.org/literature/14603832. Accessed 25 Sept. 2019.
Pollack, A. Z., et al. “Bisphenol A, Benzophenone-Type Ultraviolet Filters, and Phthalates in Relation to Uterine Leiomyoma.” Environmental Research, vol. 137, Feb. 2015, pp. 101–07. PubMed, doi:10.1016/j.envres.2014.06.028.
Qiu, Juanjuan, et al. “Association between Polymorphisms in Estrogen Metabolism Genes and Breast Cancer Development in Chinese Women.” Medicine, vol. 97, no. 47, Nov. 2018. PubMed Central, doi:10.1097/MD.0000000000013337.
—. “Association between Polymorphisms in Estrogen Metabolism Genes and Breast Cancer Development in Chinese Women.” Medicine, vol. 97, no. 47, Nov. 2018. PubMed Central, doi:10.1097/MD.0000000000013337.
Reding, Kerryn W., et al. “Genetic Polymorphisms in the Catechol Estrogen Metabolism Pathway and Breast Cancer Risk.” Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, vol. 18, no. 5, May 2009, pp. 1461–67. PubMed, doi:10.1158/1055-9965.EPI-08-0917.
Rogan, Eleanor G., et al. “Relative Imbalances in Estrogen Metabolism and Conjugation in Breast Tissue of Women with Carcinoma: Potential Biomarkers of Susceptibility to Cancer.” Carcinogenesis, vol. 24, no. 4, Apr. 2003, pp. 697–702. academic.oup.com, doi:10.1093/carcin/bgg004.
Sakhi, Amrit Kaur, et al. “Phthalate Metabolites in Norwegian Mothers and Children: Levels, Diurnal Variation and Use of Personal Care Products.” The Science of the Total Environment, vol. 599–600, Dec. 2017, pp. 1984–92. PubMed, doi:10.1016/j.scitotenv.2017.05.109.
Seachrist, Darcie D., et al. “A Review of the Carcinogenic Potential of Bisphenol A.” Reproductive Toxicology (Elmsford, N.Y.), vol. 59, Jan. 2016, pp. 167–82. PubMed Central, doi:10.1016/j.reprotox.2015.09.006.
Shao, Xiying, et al. “The CYP19 RS4646 Polymorphism IS Related to the Prognosis of Stage I–II and Operable Stage III Breast Cancer.” PLoS ONE, vol. 10, no. 3, Mar. 2015. PubMed Central, doi:10.1371/journal.pone.0121535.
Shen, Yang, et al. “Role of Single Nucleotide Polymorphisms in Estrogen-Metabolizing Enzymes and Susceptibility to Uterine Leiomyoma in Han Chinese: A Case-Control Study.” The Journal of Obstetrics and Gynaecology Research, vol. 40, no. 4, Apr. 2014, pp. 1077–84. PubMed, doi:10.1111/jog.12275.
Szaefer, Hanna, et al. “Modulation of CYP1A1, CYP1A2 and CYP1B1 Expression by Cabbage Juices and Indoles in Human Breast Cell Lines.” Nutrition and Cancer, vol. 64, no. 6, Aug. 2012, pp. 879–88. PubMed, doi:10.1080/01635581.2012.690928.
Taioli, Emanuela, et al. “Comparison of Estrogens and Estrogen Metabolites in Human Breast Tissue and Urine.” Reproductive Biology and Endocrinology : RB&E, vol. 8, Aug. 2010, p. 93. PubMed Central, doi:10.1186/1477-7827-8-93.
—. “Comparison of Estrogens and Estrogen Metabolites in Human Breast Tissue and Urine.” Reproductive Biology and Endocrinology : RB&E, vol. 8, Aug. 2010, p. 93. PubMed Central, doi:10.1186/1477-7827-8-93.
—. “Comparison of Estrogens and Estrogen Metabolites in Human Breast Tissue and Urine.” Reproductive Biology and Endocrinology : RB&E, vol. 8, Aug. 2010, p. 93. PubMed Central, doi:10.1186/1477-7827-8-93.
Thayer, Kristina A., et al. “Bisphenol A, Bisphenol S, and 4-HydroXyphenyl 4-IsoproOxyphenylSulfone (BPSIP) in Urine and Blood of Cashiers.” Environmental Health Perspectives, vol. 124, no. 4, Apr. 2016, pp. 437–44. PubMed, doi:10.1289/ehp.1409427.
Thomson, Cynthia A., et al. “A Randomized, Placebo-Controlled Trial of Diindolylmethane for Breast Cancer Biomarker Modulation in Patients Taking Tamoxifen.” Breast Cancer Research and Treatment, vol. 165, no. 1, Aug. 2017, pp. 97–107. PubMed, doi:10.1007/s10549-017-4292-7.
Vandermarken, T., et al. “Assessment of Estrogenic Compounds in Paperboard for Dry Food Packaging with the ERE-CALUX Bioassay.” Chemosphere, vol. 221, Apr. 2019, pp. 99–106. PubMed, doi:10.1016/j.chemosphere.2018.12.192.
Wang, Kai-Hung, et al. “Bisphenol A at Environmentally Relevant Doses Induces Cyclooxygenase-2 Expression and Promotes Invasion of Human Mesenchymal Stem Cells Derived from Uterine Myoma Tissue.” Taiwanese Journal of Obstetrics & Gynecology, vol. 52, no. 2, June 2013, pp. 246–52. PubMed, doi:10.1016/j.tjog.2013.04.016.
Wanwimolruk, Sompon, and Virapong Prachayasittikul. “Cytochrome P450 Enzyme Mediated Herbal Drug Interactions (Part 1).” EXCLI Journal, vol. 13, Apr. 2014, pp. 347–91.
Wielsøe, Maria, et al. “Genetic Variations, Exposure to Persistent Organic Pollutants and Breast Cancer Risk – A Greenlandic Case-Control Study.” Basic & Clinical Pharmacology & Toxicology, vol. 123, no. 3, Sept. 2018, pp. 335–46. PubMed, doi:10.1111/bcpt.13002.
Yang, Li, et al. “Novel Biomarkers for Risk of Prostate Cancer: Results from a Case–Control Study.” The Prostate, vol. 69, no. 1, Jan. 2009, pp. 41–48. onlinelibrary.wiley.com, doi:10.1002/pros.20850.
Ye, Yi, et al. “CYP1A1 and CYP1B1 Genetic Polymorphisms and Uterine Leiomyoma Risk in Chinese Women.” Journal of Assisted Reproduction and Genetics, vol. 25, no. 8, Aug. 2008, pp. 389–94. PubMed Central, doi:10.1007/s10815-008-9246-x.
—. “CYP1A1 and CYP1B1 Genetic Polymorphisms and Uterine Leiomyoma Risk in Chinese Women.” Journal of Assisted Reproduction and Genetics, vol. 25, no. 8, Aug. 2008, pp. 389–94. PubMed Central, doi:10.1007/s10815-008-9246-x.
Yu, Hongping, et al. “A Functional NQO1 609C>T Polymorphism and Risk of Gastrointestinal Cancers: A Meta-Analysis.” PLoS ONE, vol. 7, no. 1, Jan. 2012. PubMed Central, doi:10.1371/journal.pone.0030566.
Zahid, Muhammad, et al. “Unbalanced Estrogen Metabolism in Ovarian Cancer.” International Journal of Cancer. Journal International Du Cancer, vol. 134, no. 10, May 2014, pp. 2414–23. PubMed Central, doi:10.1002/ijc.28565.
—. “Unbalanced Estrogen Metabolism in Ovarian Cancer.” International Journal of Cancer. Journal International Du Cancer, vol. 134, no. 10, May 2014, pp. 2414–23. PubMed Central, doi:10.1002/ijc.28565.
Zhang, Yixiang, et al. “Association between GSTP1 Ile105Val Polymorphism and Urinary System Cancer Risk: Evidence from 51 Studies.” OncoTargets and Therapy, vol. 9, 2016, pp. 3565–69. PubMed, doi:10.2147/OTT.S106527.