Key takeaways:
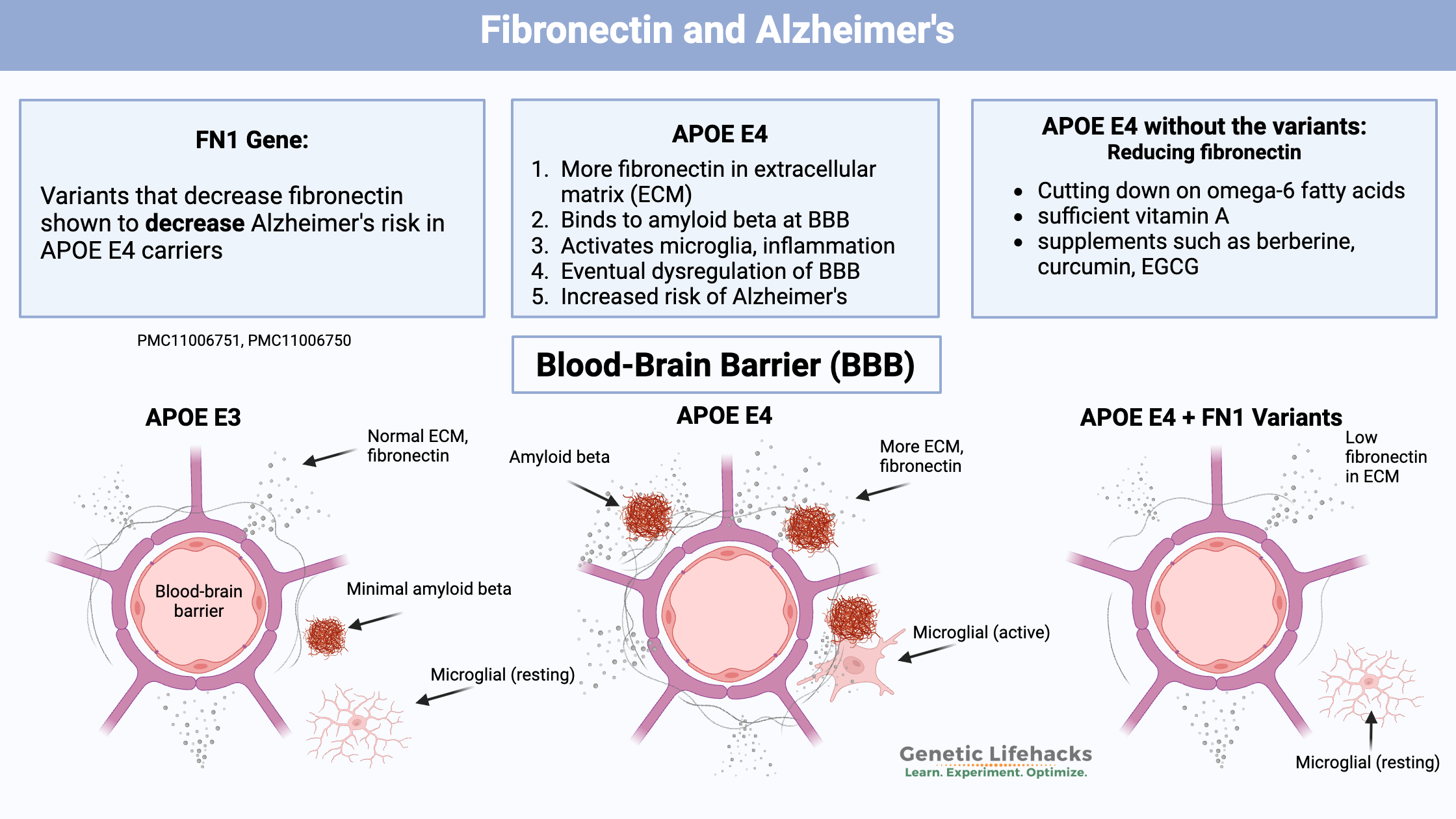
~ Two new studies identify genetic variants in FN1, which encodes fibronectin, as being protective against Alzheimer’s in people with APOE E4.
~ Fibronectin interacts with amyloid-beta and the blood-brain barrier.
~ Variants that reduce fibronectin decreased Alzheimer’s risk by 70%.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
Alzheimer’s pathology due to BBB dysfunction:
The biggest risk factor for late-onset Alzheimer’s disease is carrying an APOE E4 allele. For several decades, Alzheimer’s research has focused on preventing the buildup of amyloid-beta plaque in the brain. While amyloid-beta plaque clearly plays a role in the pathogenesis of Alzheimer’s disease, the drugs developed to block amyloid-beta haven’t helped much with Alzheimer’s symptoms. At best, they may slow the progression of the disease.
Amyloid-beta plaques are thought to be deposited in response to activation of microglia (cells of the brain’s immune system). Inflammation and oxidative stress in the brain activate microglia. But another part of the picture in Alzheimer’s pathology is changes in the blood vessels – the cerebral vasculature and the blood-brain barrier.
Recently, two large studies of the interaction of APOE E4 with other genetic variants have shed light on how changes in the blood vessels and the blood-brain barrier are important for the buildup of amyloid-beta plaques.[ref][ref]
Both genetic studies found that variants that reduce fibronectin significantly decreased the risk of Alzheimer’s in people with APOE E4 genotypes. The variants identified were not very common (around 1-2% of the population).
Personally, I’m excited about this research, not just for the 1% of people with the variant, but because it strongly suggests what goes wrong in the brain to cause Alzheimer’s. Most importantly, this is a potentially modifiable pathway.
Let’s look at what’s going on with fibronectin, the blood-brain barrier, and Alzheimer’s. Then I’ll include the variants in the genotype report that are associated with reduced Alzheimer’s risk in APOE E4 carriers. (You can check your APOE E4 genotype here — if you want to know.)
Fibronectin and Alzheimer’s:
Fibronectin is an adhesive glycoprotein found in the extracellular matrix and in the blood plasma. Many people think of fibronectin in relation to blood clots, and it plays an important role in clotting. However, there is more to fibronectin than its role in the extracellular matrix and clotting. It also is important in immune response, cellular adhesion, and the blood-brain barrier.
The extracellular matrix is a network of proteins that surrounds and supports cells. Within the extracellular matrix, fibronectin helps by binding to proteins such as fibrin, collagen, and integrins on the surface of cells.
Fibronectin also plays a role in cell adhesion, immune response, and the blood-brain barrier. Important to Alzheimer’s, fibronectin also interacts and binds with amyloid-beta as well as inflammatory cells in the brain.
The blood-brain barrier in Alzheimer’s:
The blood-brain barrier (BBB) is compromised in Alzheimer’s disease, and research shows that the changes in the BBB occur early in the progression of the disease.[ref] The blood-brain barrier not only keeps bacteria and viruses out of the brain, but it also keeps out many molecules that normally circulate throughout the body. The brain is protected and prioritized, with certain molecules being kept in the brain and many molecules being filtered out.
As mentioned above, fibronectin is integral to the structure of the blood-brain barrier. In people with APOE E4 and Alzheimer’s, there is increased fibronectin in the blood-brain barrier. One study showed that compared to people with APOE ε3/3, fibronectin expression increased by 8.1% in people with one copy of APOE E4. In individuals with two E4 alleles, there was a 26.6% increase in fibronectin in the blood-brain barrier. A specific type of collagen is also increased in APOE E4 in the blood-brain barrier.[ref]
Two things are likely going on here:
- increased amyloid-beta trapped in the brain
— which leads to — - eventual BBB dysfunction.
Stick with me, it gets a bit complicated.
1. Increased amyloid-beta in the brain and inflammation:
In Alzheimer’s, the buildup of amyloid-beta in the brain activates the immune system, causes inflammation, and eventually kills neurons.
Individuals with the APOE E4 allele don’t break down amyloid-beta quite as well as normal for clearance from the brain. Fibronectin interacts with and binds to many different particles, including amyloid-beta. The excess fibronectin in the BBB is believed to “negatively regulate amyloid beta clearance”.[ref]
Essentially, fibronectin in the blood-brain barrier is binding with amyloid-beta. Normally, a lot of the amyloid-beta is cleared out of the brain and circulates in the bloodstream in a soluble form.
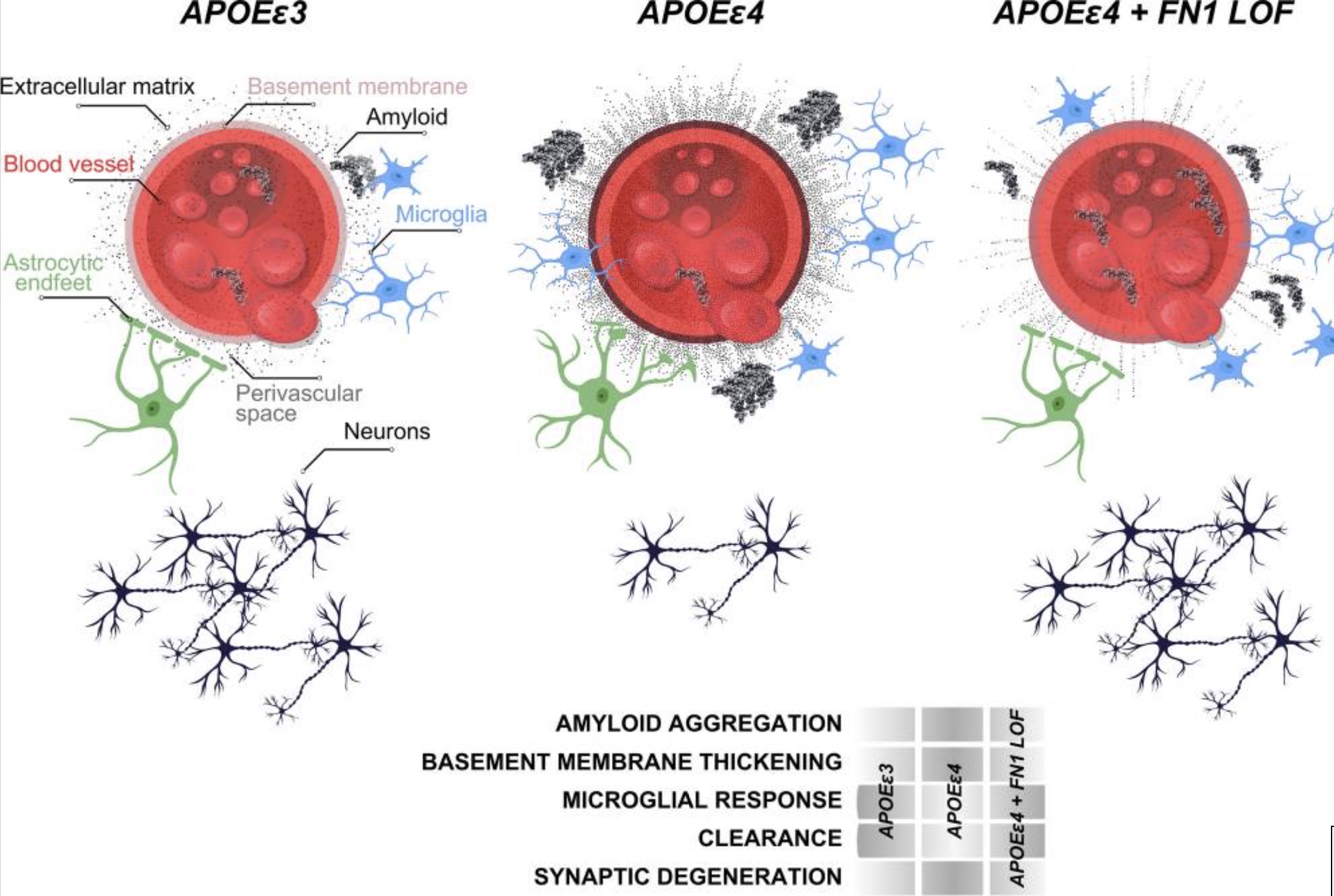
2. Damaged blood-brain barrier (BBB):
The interaction between fibronectin in the ECM (extracellular matrix) of the blood-brain barrier and amyloid-beta exacerbates the role of fibronectin and its attachments to immune cells. The chronic attachment of fibronectin in the ECM to immune cells then “damages the vascular functions, contribute to BBB breakdown, and loss of synaptic integrity.”[ref]
I found this confusing at first, thinking that more fibronectin with APOE E4 would lead to better blood-brain barrier function. However, the interactions that chronically trigger immune cells eventually lead to BBB dysfunction.
Multiple previous studies also point to a causal role of BBB changes in Alzheimer’s disease. For example, a 2019 study showed that blood-brain barrier abnormalities occur before, or even independently of, amyloid-beta and/or tau deposition.[ref]
Why is BBB dysfunction a problem?
One reason is that the disruption of the BBB in the early stages of Alzheimer’s allows fibrinogen to enter the brain. Fibrinogen is another coagulation or clotting protein, and excess fibrinogen also interacts with amyloid beta to form the plaques in Alzheimer’s disease.[ref]
Fibrinogen is a plasma protein that can be activated to form fibrin, which sticks together with other molecules to form blood clots. It is normally blocked from the brain by the BBB and isn’t found in the cerebrospinal fluid of healthy individuals.
The fibronectin/APOE4 connection is just one way that the BBB can be disrupted.
Traumatic brain injury (TBI, concussions) that disrupts the BBB also allows fibrinogen into the brain. In both TBI and neurological disease, fibrinogen in the brain plays a causative role in the degeneration. Fibrinogen can bind to receptors expressed by microglia and macrophages, and it can also bind to amyloid-beta, causing clumping.[ref]
Genetic studies on Alzheimer’s and fibronectin or fibrinogen:
As I mentioned earlier, two new genome-wide studies looked at the intersection of APOE E4 carriers, Alzheimer’s diagnosis, and other genes. Both studies identified FN1 gene variants, which cause decreased fibronectin, as protective against Alzheimer’s in people with APOE4 alleles.[ref][ref]
The first study went beyond just the statistical analysis of the genetic variants. The researchers also used an animal model with reduced FN1 to determine how and why reduced fibronectin would also reduce inflammation in the brain. The second study (still in preprint) was able to replicate the genetic findings in other Alzheimer’s data sets.
Previous studies have also found that fibronectin levels are higher in people with Alzheimer’s disease, and the interaction between fibronectin and amyloid-beta has been shown in cell studies.[ref][ref]
What about fibrinogen genes?
Mendelian randomization studies are used to determine causality vs. association. Fibrinogen and γ-fibrinogen levels are elevated in Alzheimer’s, Parkinson’s, and Lewy body dementia. However, using a Mendelian randomization method, researchers found that there was no causal relationship between higher fibrinogen levels and the risk of neurodegenerative diseases.[ref]
Related article: Fibrinogen genetic variants and clotting risk
Genotype report:
Lifehacks:
According to one of the researchers in the study: “Anything that reduces excess fibronectin should provide some protection, and a drug that does this could be a significant step forward in the fight against this debilitating condition,” Kizil said.[ref]
6 Natural Ways to Inhibit Fibronectin and Potential AD Prevention
Given the role of fibronectin in AD pathology, reducing its levels may be a therapeutic avenue.
As always, please talk with your doctor before starting any supplements if you have medical questions or are concerned about medication interactions. Keep in mind that fibronectin is also important for wound healing and clot formation.
Here are some natural ways to potentially lower fibronectin levels:
Reducing omega-6 oils:
Studies show that omega-6 fatty acids increase fibronectin levels.[ref]
“Vegetable oils” such as canola, corn, soybean, sunflower, and safflower oils are high in omega-6 fatty acids. Most fried foods, sauces, and processed foods are very high in omega-6 fatty acids. Meat from animals that are fed a lot of omega-6 fats, such as chickens and pigs, can also be high in omega-6.
One of the biggest changes in our eating habits over the past 70 years is the huge increase in omega-6 fatty acids. Here’s a chart showing the change in consumption of types of fats since 1909. The gray line shows omega-6-rich vegetable oils.
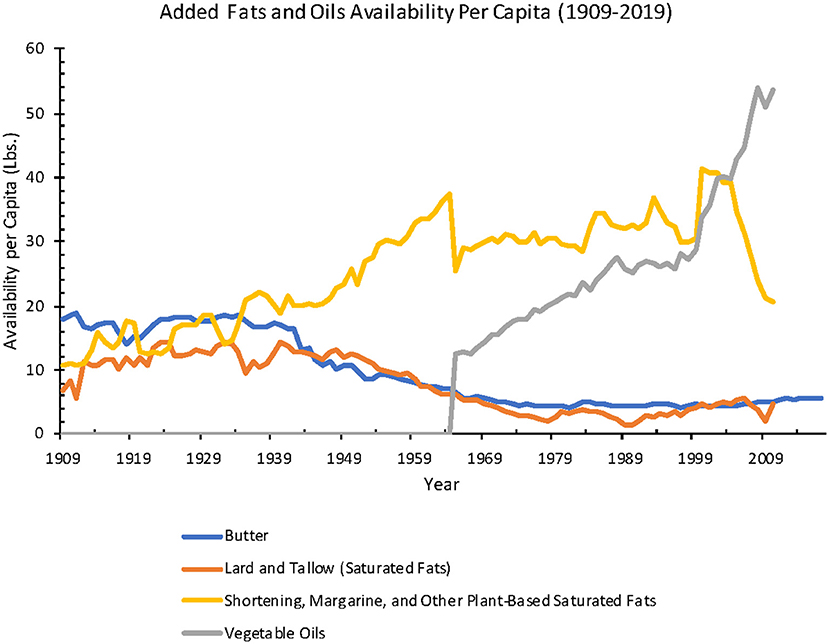
Many prior studies have also made the connection between omega-6 fatty acids and increased risk of Alzheimer’s in animals and in cell studies, but the published research on humans has been a lot more ambiguous.[ref][ref][ref]
Omega-3 fatty acids, such as DHA and EPA from fish and seafood, do not increase fibronectin levels.[ref] One study found that fibronectin decreased when omega-3 was increased in the diet.[ref]
Reducing omega-6 fatty acids is difficult with a modern diet. Check the labels of what you normally eat and you’ll likely find that the fat comes from sources high in omega-6. In addition to fried foods and chips, salad dressing, mayonnaise, and other sauces often contain quite a bit of omega-6 oil.
Vitamin A:
Animal studies show that vitamin A deficiency leads to higher serum fibronectin levels.[ref] Vitamin A in the retinol form is important in turning on or off gene transcription for a number of different genes, including several related to the extracellular matrix.[ref]
The best way to know if you are deficient in vitamin A is to get a blood test. In most states in the US, you can order the test yourself (if you don’t want to go through your doctor).
If you want to increase your vitamin A levels, there are two options: pre-vitamin A from beta-carotene (plants) or retinol forms of vitamin A (animal foods). Some people have genetic variants that make it harder for them to convert beta-carotene into the active retinol form, so you may want to check your vitamin A genes to see whether beta-carotene is a good option to rely on for you.
Related articles and topics:
Alzheimer’s Gene: Find your APOE type from your genetic raw data