Key takeaways:
~ Researchers first discovered FTO as a gene associated with higher BMI and obesity.
~ More recently, the function of FTO was found to be a m6A methyl eraser, affecting the gene expression of thousands of genes, including those involved in fat storage, cancer, neurodegeneration, viral response, heart disease, and more.
~ As a core regulator of gene expression, the FTO gene variants impact much more than just weight. This may explain the association of obesity with cancer, heart disease, neurodegeneration, autoimmune diseases, and infectious disease response.
Regulating gene expression: FTO and mRNA methylation
The FTO gene was first identified as an obesity-related gene due to FTO variants that are statistically linked to increased BMI. Researchers called it the fatso gene, due to the large size of the gene (and probably a nerdy sense of humor).[ref][ref] The FTO abbreviation comes from the initial discovery of the gene in the fused toe (Ft) mice.
Currently, there are thousands of studies on how FTO gene variants increase the statistical risk of obesity and more generally is associated with increased average BMI. It is the strongest genetic component of increased BMI and weight.
Related article: FTO and weight
However, the focus on weight and obesity with the FTO gene misses the boat!
Researchers figured out more than a decade ago that the FTO gene encodes a protein that regulates gene expression of thousands of genes, including some related to fat storage.[ref]
The FTO protein acts on what is called m6A methyl markers which control how mRNA is turned into its protein. Essentially, FTO regulates the expression of genes related to cancer, circadian rhythm, adult neurogenesis, fat storage, and even the replication of viruses in an infection.
Let’s dig into the research on how FTO controls gene expression, how it affects cancer, neurodegeneration, infectious disease susceptibility, and so much more.
Along the way, think about how obesity is statistically linked to increased cancer, neurodegeneration, infectious diseases, etc… The way FTO controls gene expression in all of those areas gives rise to whether obesity increases the risk of those diseases or whether the FTO variant increases the risk. This is an important distinction because not everyone with the FTO variants is obese, and it could be a risk factor that is being missed- hiding behind the epidemiological connection of obesity.
First, a quick overview of some basic biology on mRNA to make sure we are all on the same page. Skip ahead if you know this. Next, I’ll explain the recent research on mRNA m6A methylation and gene expression. Finally, I’ll cover the FTO genetic variants in depth.
What is mRNA? Overview of gene -> mRNA -> protein
I often state that a gene codes for a protein – and that is true in a general sense.
Genes are segments of DNA that are transcribed into mRNA (messenger RNA). Ribosomes then translate the mRNA into the amino acids that make up proteins.
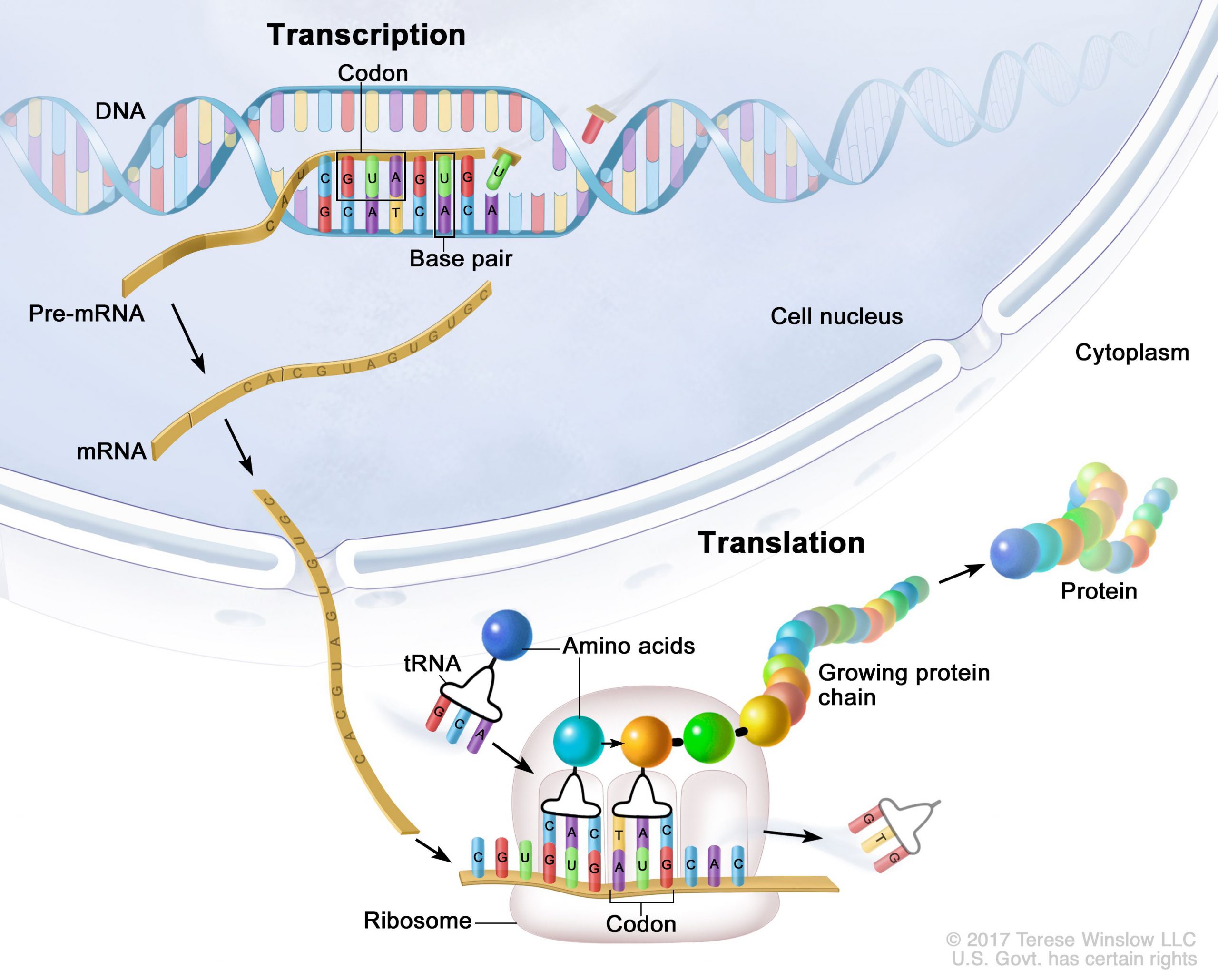
Sounds pretty straightforward… but there is a lot more going on inside the cell.
All the nucleated cells in your body have the same DNA and the same genes. (The exception is red blood cells, which don’t have a nucleus or nuclear DNA.)
However, not every gene in every cell is going to be turned into its protein. For example, the cells in your liver function way differently than your brain cells. In liver cells, specific genes are translated into proteins and enzymes, while other genes are going to be active in the neurons of the brain.
Therefore, while your cells all have the same DNA instructions for making proteins, not all genes will be transcribed and translated into proteins.
Epigenetics is a term that refers to the process by which genes are turned ‘on or off’ as a way of controlling gene expression. There are multiple ways of marking genes to be turned on for transcription and translation or to be turned off and not transcribed or not translated. Gene expression can also be controlled after the mRNA is translated into the protein, which is called post-translational modification.
You may have read about the methylation of DNA in reference to MTHFR. Methyl groups can attach to DNA in the nucleus and prevent it from being transcribed into a protein. That can also happen to mRNA.
Once the mRNA copy of a gene is created, it moves out of the cell nucleus and into the cytosol for translation into a protein by the ribosomes. Along the way, there are a bunch of ways that the mRNA can be modified so that it can’t complete the steps needed to be turned into the protein. It can also be modified or spliced to produce slightly different proteins or enzymes. There are ways to modify the mRNA so that it facilitates translation into the protein more quickly. MicroRNAs can attach to the mRNA to prevent translation.
Just like methyl markers can turn off transcription of a gene, methyl markers can be added to specific spots on the mRNA to stop or alter translation. The most common way of regulating gene expression in animals, plants, and bacteria is through m6A methylation.[ref]
This last option – m6A methyl markers on mRNA – is where FTO comes in.
Adding a methyl marker to mRNA: writers
One of the most common ways that mRNA is altered is through methylation markers attaching at specific spots on the mRNA.
The most common spot on the mRNA is a certain adenosine molecule that a methyl group can be attached to. This is called N6-methyladenosine (m6A) modification.
Essentially, a methyl group (a carbon plus three hydrogen, CH3) is added to the m6A spot on the mRNA. Methyl groups are used in cells in a bunch of ways, and stopping the conversion of mRNA into a protein is just one use.
Related article: Methylation cycle, methyl groups, and MTHFR
What does this methylation mark do?[ref]
- The m6A methylation can cause the mRNA for a protein to be unstable and then degraded.
- Or, it can cause it to remain in the nucleus and not move into the cytosol to become a protein at that particular time.
- It can alter the way that the mRNA forms the protein.
- Additionally, a methyl group in the right spot on the mRNA can increase stability and promote translation.
So we have methyl marks controlling gene expression in a bunch of ways.
What causes m6A methylation?
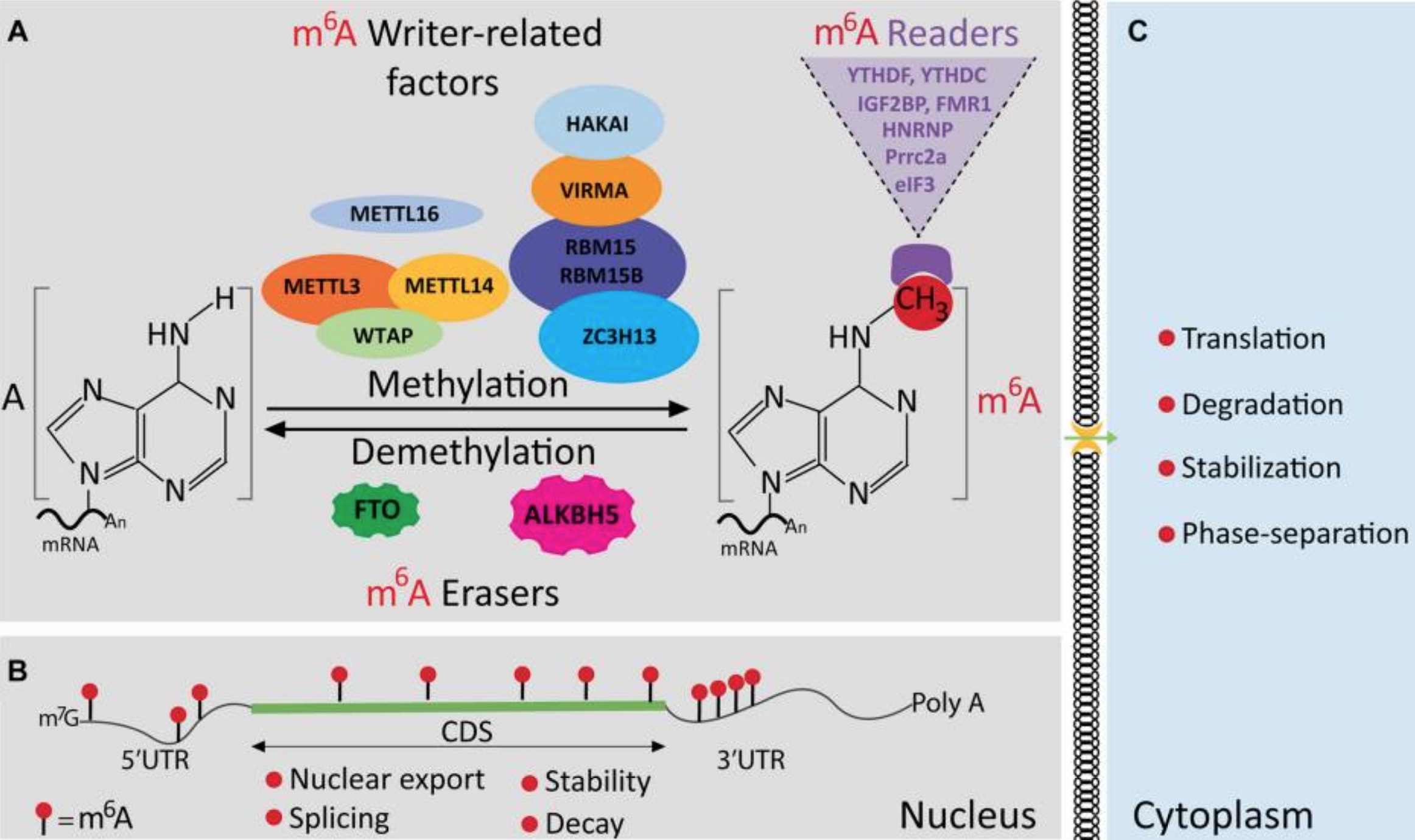
The methyl group is added to mRNA by proteins (enzymes) called M6A writers. The two most common are METTL3 and METTL14.
The METTL3 and METTL14 enzymes ‘write’ or attach the methyl group onto the mRNA at specific spots. These m6A writers, called RNA methyltransferases, are enzymes that transfer a methyl group to an RNA.[ref]
The METTL3 writer and m6A methylation are an essential part of embryonic development. Without METTL3, a fetus is not viable.[ref] METTL14 is essential in modulating gene expression for many different biological pathways. It’s important in cancer, and it is also important in heart health. For example, exercise reduces METTL14 levels which allows for the growth of cardiac muscle cells.[ref]
Enough with writers – let’s get on to the main topic, FTO and erasing the mRNA methyl markers.
Erasing the m6A markers: FTO
Marking the mRNA with a methyl group stops the translation into the protein, and the cell needs a way to resume that protein translation when it is needed in the cell — a way to flip the ‘off’ switch back to on.
Methyl writers are adding the methyl groups, and eraser proteins remove the m6A methylation. These ‘erasers’ are called m6A demethylases (enzymes that remove the methyl group from m6A).
In some situations, erasing the m6A methylation then allows the mRNA to become the protein. The eraser can ‘turn up the volume’ of the protein in this way. In other situations, erasing the methyl group can alter the stability of the mRNA. So this isn’t always like flipping the ‘on’ switch.
Researchers have found two main m6A erasers: ALKBH5 and FTO.
Researchers discovered that the FTO gene strongly correlated with higher BMI and obesity in 2007. More recently, researchers discovered that FTO is an m6A eraser that removes methyl groups from mRNA. This discovery has opened up huge avenues of research on topics from cancer to immune response to heart disease.
Reading the m6A markers:
There is one more player in the mRNA methylation game: the readers. These reader enzymes are responsible for degrading the methyl-marked mRNA so that it doesn’t complete the cycle of becoming a protein. The reader proteins include YTHDF2, YTHDF1, and IGF2BP.
One article sums up: “In short, writers and erasers work together to modulate m6A dynamics and maintain its homeostasis in cells, while the activity of readers allows m6A to exert its influence in each step of the RNA life cycle.”[ref]
Here is a visual overview of the different outcomes from m6A readers:[ref]
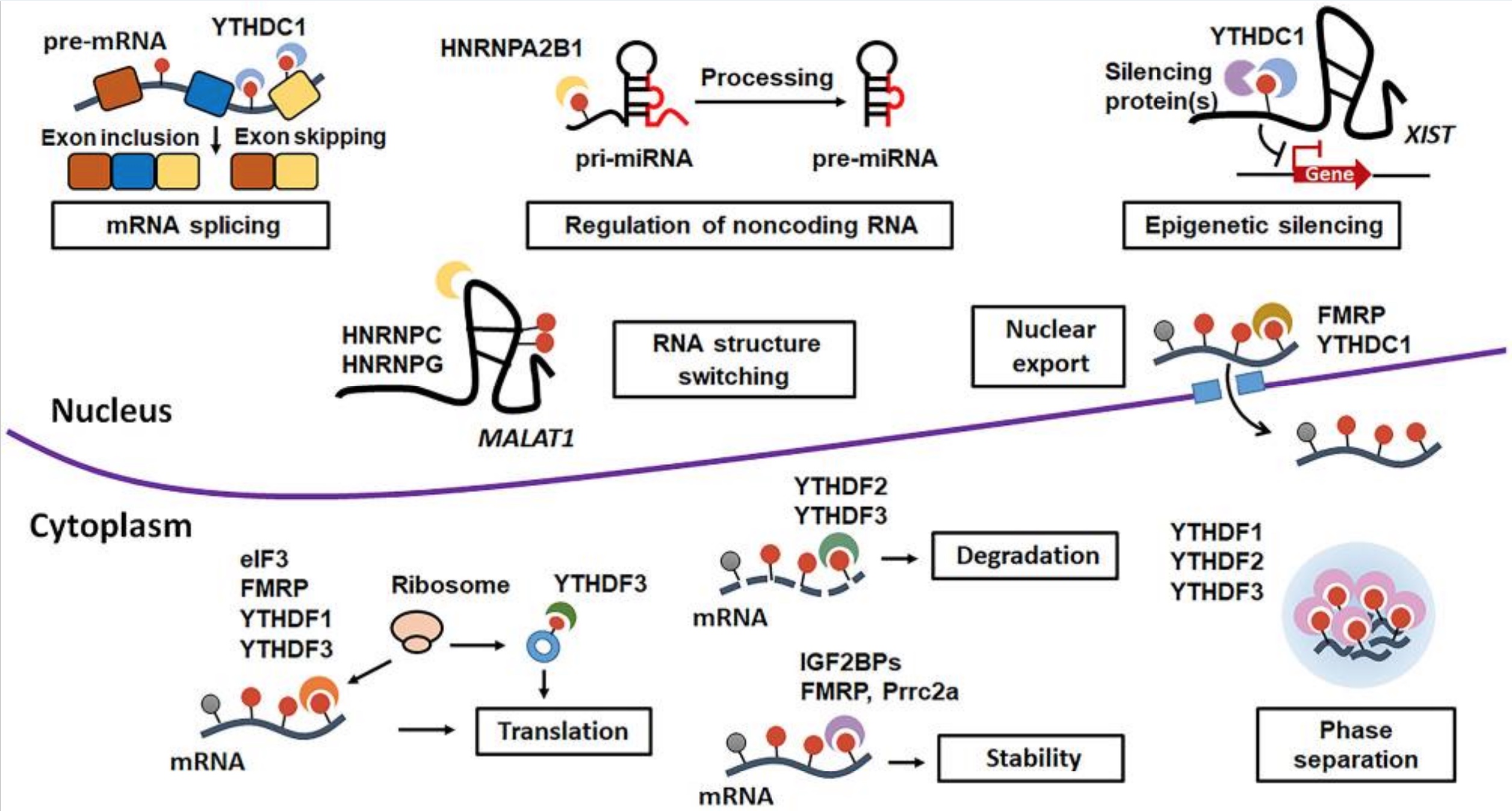
Recap: Genes are transcribed into mRNA. The mRNA can be translated into a protein, or it can be marked with methyl groups in a way that alters it (splices it, causes it to degrade, moves it to the cytosol, or stabilizes it for translation). FTO is an eraser of those marks.
What does the FTO gene impact?
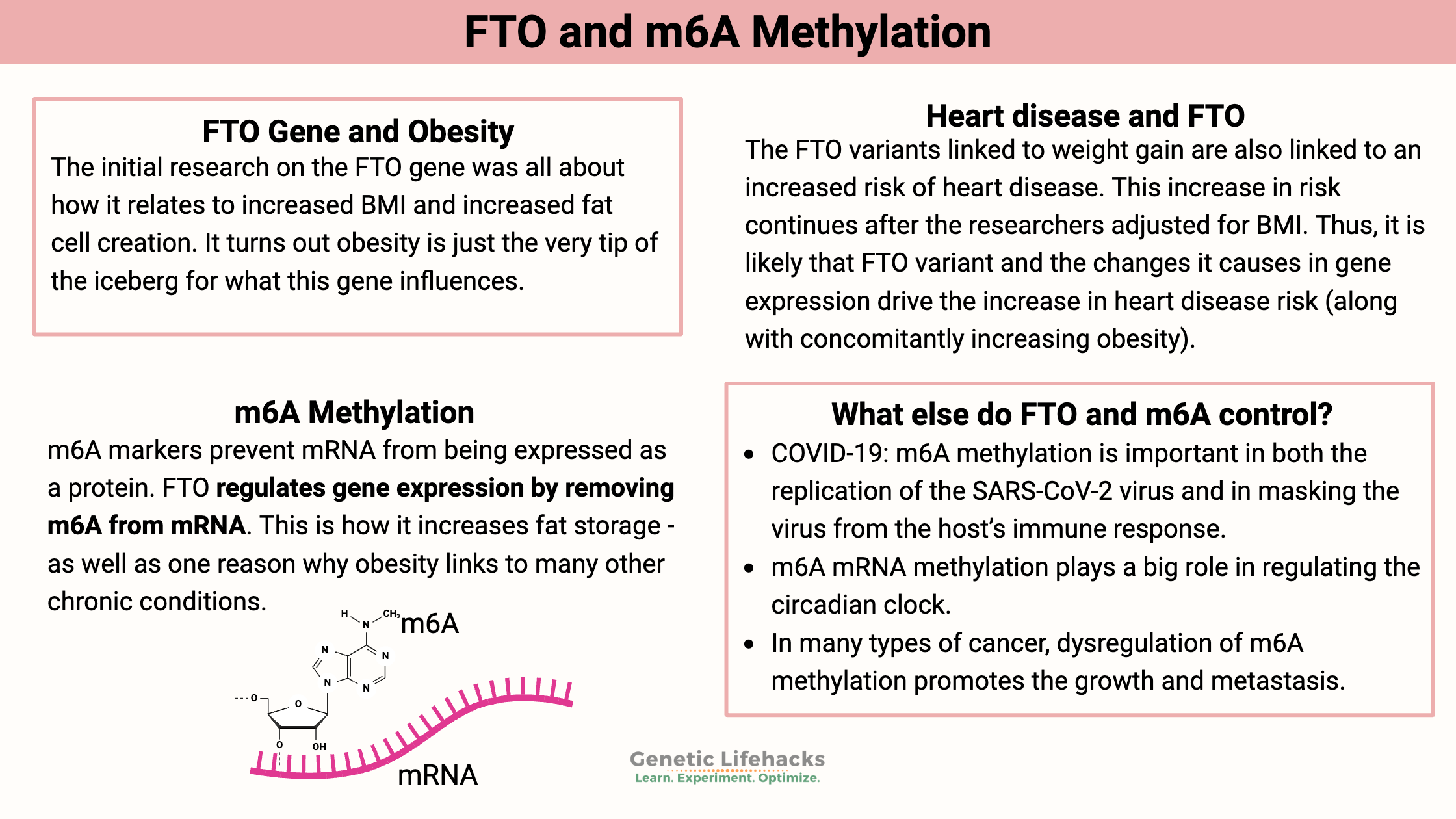
The initial research on the FTO gene was all about how it relates to increased BMI and increased fat cell creation. It turns out obesity is just the tip of the iceberg for what this gene influences.
m6A methylation and FTO impact:
- cancer
- circadian rhythm
- neurogenesis, cognitive function, and Alzheimer’s disease
- viral replication in infections such as Covid
- heart health
- fat storage
- diabetes complications
Let’s dig into the latest research on FTO as an m6A eraser:
Cancer and FTO
Much of the current research on m6A methylation of mRNA focuses on cancer. In many types of cancer, dysregulation of m6A methylation promotes the growth and metastasis of cancer.
As an oversimplification, cancer mutations cause either too much cell growth or promote metastasis. The key is to prevent both.
Dysregulation can mean too much of a methylation writer on certain mRNA, too many or not enough methyl groups available, or too much of the eraser.[ref][ref]
Two ways that dysregulation of m6A methylation impacts cancer are:
- oncogenes can be activated to promote unchecked growth – or –
- tumor suppressor genes can be repressed via m6A methylation.
Thus, more FTO can be beneficial and inhibit certain cancers, but in other tumors it can promote growth.
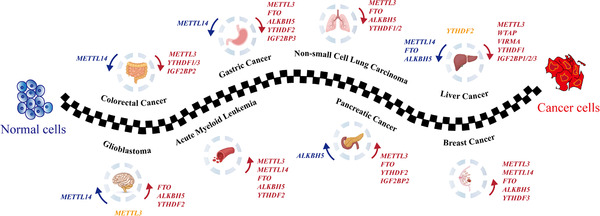
Here are a few of the recent discoveries related to higher FTO and cancer:
- In gastric cancer, higher levels of FTO are an independent risk factor for overall survival. FTO promotes metastasis by erasing m6A methylation marks on certain mRNAs (Integrin β1).[ref]
- FTO promotes tumor progression in esophageal squamous cell cancer and colon cancer.[ref]
- A Japanese study shows that the FTO variants have associations with an increased relative risk of pancreatic cancer independent of obesity.[ref]
- Inhibiting FTO may be a new target for cancer therapy in leukemia.[ref]
It isn’t as simple as more FTO is bad and less is good.
- Another study on FTO in colon cancer showed that the hypoxia present in the tumor suppressed FTO, and the FTO could also exert a tumor-suppressive role by modifying certain mRNAs.[ref]
- The FTO genetic variants that increase FTO expression have links to a significant decrease in the risk of prostate cancer.[ref][ref]
- FTO may play a preventative role in liver cancer and prevent the progression of thyroid cancer.[ref][ref]
The Circadian Clock is controlled by m6A methylation
Your body has a built-in circadian rhythm controlled by a molecular clock. The circadian rhythm controls when different activities happen in the body – the rhythm of hormones, body temperature, enzyme production, and even sleep.
The circadian ‘clock’ is a molecular one instead of a mechanical clock with gears. The rise and fall of core circadian proteins (CLOCK and BMAL1 vs. PER and CRY) control the circadian rhythm throughout the body.
Related articles: BMAL1, Circadian rhythm and weight, Circadian rhythm and depression
Researchers recently discovered that m6A mRNA methylation plays a big role in the circadian clock.[ref]
Specifically, FTO is responsible for controlling the levels of the core circadian clock genes CLOCK and BMAL1. [ref] m6A methylation is also responsible for downstream lipid production in the liver via circadian regulation.[ref]
Food for thought: Are the studies linking circadian rhythm disruption and weight gain, cancer, or depression risk taking into account the impact of FTO variants?
Neurogenesis and neurodegeneration:
Adult neurogenesis refers to the continual (slow) replacement of neurons in the brain from neuronal stem cells.
Depending on your age, you may have learned, like I did, that you can’t make new brain cells. (Was this why your brain on drugs looks like a fried egg? perhaps I’m misremembering the commercials on that).
It turns out the ‘you can’t make new brain cells’ part of my high school textbook was wrong. Instead, there is lifelong neurogenesis in all mammals, including humans.[ref]
Recent research (2021) shows that FTO is important in adult neurogenesis as well as learning and memory.
Researchers found that reducing the FTO protein in the brains of animals caused impaired learning and memory.[ref]
Other research shows that FTO regulates the expression of BDNF, brain-derived neurotrophic factor, which is important in brain plasticity, cognitive function, mood disorders, and dementia.[ref]
Related article: BDNF genetic variants
It isn’t as simple as FTO is always “good” in the brain. Instead, FTO has many different effects and interacts with many other genes. Additionally, keep in mind that FTO is the eraser, so the m6A methylation marks (methyl groups) need to be added by the writer in the first place for FTO to matter.
The FTO variant that increases the amount of FTO correlates to an increased risk of Alzheimer’s only in APOE E4 carriers.[ref]
In Parkinson’s disease, an overall decrease in m6A methylation exists.[ref] Early animal research points to inhibiting FTO as having the potential to protect the dopamine neurons that are targeted in Parkinson’s.[ref]
A recent study found that children with the FTO variant linked to obesity also have greater brain volume in certain regions.[ref]
Heart disease and FTO:
Research shows that lower FTO expression prevents vascular inflammation, while higher FTO expression increases cellular senescence and inflammation in endothelial cells in aging.[ref] Vascular inflammation and cellular senescence in the endothelial cells are one driver of heart disease.
The genetic variants linked to increased FTO (listed below) are also linked to an increased risk of heart disease. This increase in risk is after the researchers adjusted for things like sex, age, and BMI. Thus, it is likely that being overweight wasn’t the (only) cause of the increase in heart disease risk. Instead, it was the increased FTO m6A eraser.[ref]
However, some Mendelian randomization studies use the FTO variants and their association with obesity as a way of showing that heart disease is caused by obesity.[ref] One drawback of Mendelian randomization studies is that the FTO gene variants are often used to prove causality for obesity.
It’s not as straightforward, though, as high FTO levels cause all the negatives seen in heart disease. In mice, decreasing FTO expression causes an increase in atherosclerotic plaques. The opposite was also true – increasing FTO expression inhibited atherosclerosis.[ref] Similarly, in mouse models of heart attack, increasing FTO was beneficial in decreased fibrosis and enhanced angiogenesis.[ref]
Viral RNA replication: m6A methylation and FTO
Viruses hijack the host cell’s DNA and RNA replication pathways to replicate the virus.
RNA viruses, or at least some RNA viruses, incorporate the m6A methylation writers and erasers to modify both the viral RNA and the host’s immune response. Also, m6A methylation impacts DNA viruses.[ref] Another thing that m6A methylation does is help the innate immune system figure out viral RNA and human mRNA. The innate immune response has to identify a viral RNA from a normal ‘self’ mRNA. One way that a virus can ‘hide’ from the host’s immune response is through m6A methylation at just the right spot on the viral RNA.[ref]
While m6A has been known since the 1970s, it was only a few years ago that technological breakthroughs allowed researchers to understand the role of viruses. Here are some of the viruses that interact with FTO and m6A methylation:
- Porcine endemic diarrhea virus (PEDV), an aptly named coronavirus, gives pigs deadly diarrhea. Researchers recently investigated the interaction between m6A methylation and PEDV replication. The results showed that silencing FTO decreased viral replication.[ref]
- HIV and Zika’s viral replication are also regulated by m6A modifications. Silencing or reducing the m6A erasers, including FTO, can decrease the replication of the Zika virus.[ref]
- The host response is modified by m6A methylation in HIV. The m6A methylation on certain spots of HIV RNA causes the host’s interferon response to be inhibited. Knocking out the FTO eraser decreased the host interferon response significantly.[ref]
- People with genetic variants that increase FTO have a lower likelihood of response to hepatitis C therapy. Confounding factors here include the FTO variant carriers having a higher BMI and possibly liver fat.[ref]
- Other viruses that interact with m6A methylation include hepatitis C, hepatitis B, influenza A, Kaposi’s sarcoma associate virus, and SV40.[ref]
m6A methylation and COVID-19
FTO and m6A methylation are important in Covid. (Obesity is a risk factor for covid, and some in public health recommend weight loss to prevent covid. Again, if it is FTO variants instead of obesity, then weight loss is not going to change FTO levels.)
An early 2020 research paper showed that m6A methylation on the spike protein may be one of the structural and functional differences between the original SARS and SARS-CoV-2. The SARS-CoV-2 spike protein binds to the ACE2 receptor more effectively.[ref]
Preventing the immune system from seeing the virus:
m6A methylation is important in both the replication of the SARS-CoV-2 virus and in masking the virus from the host’s immune response.[ref]
SARS-CoV-2 replication:
A paper from the Wuhan Institute of Virology also showed that m6A methylation is important in how SARS-CoV-2 replicates. The researchers concluded that the knockdown of the METTL3 (writer) methylation caused a decrease in SARS-CoV-2 replication.[ref] Research from Peking University also found that m6A methylation is important in SARS-CoV-2 replication. Essentially, they also conclude that reducing the writing of methyl marks on m6A decreased the replication of SARS-CoV-2.[ref]
Researchers at UC San Diego decided to modify the m6A binding site by generating mutations to see what the effect would be on the SARS-CoV-2 virus. Generated mutations in the m6A binding sites that disrupted binding caused an increase in the host immune response (i.e., the virus was no longer as hidden from the immune response).[ref]
Rabbit trail: methylation, methyl groups, and Covid
Methylation is dependent on the availability of methyl groups. Folate and choline are the two main dietary sources of methyl groups. Researchers from the Broad Institute have also shown that SARS-CoV-2 replication is dependent on folate and methyl groups (aka one-carbon metabolism). They found that depleting cellular folate and, specifically, the production of methyl groups inhibited viral replication.[ref]
One way to limit methyl groups is via anti-folate drugs, which are often used for chemotherapy. It turns out that methotrexate, an anti-folate drug, inhibits the replication of SARS-CoV-2. Proguanil, an antifolate drug for malaria, and sulfasalazine, another antifolate drug, seem to inhibit the replication of SARS-CoV-2.[ref][ref]
Going off on a tangent:
A lot of countries have mandated the folic acid fortification of cereal grains which increases the availability of methyl groups. This is done as a public health initiative for the prevention of folate deficiency in pregnant women, but the result is that the whole population gets large doses of folic acid when eating bread, white rice, tortillas, baked goods, and breakfast cereal. The areas of the world with folic acid fortification in the food supply overlap with the areas with higher COVID deaths. This could be due to folic acid increasing methyl groups – or it could be due to other things, like something else in processed foods.
FTO: Obesity and fat storage
Higher levels of FTO due to genetic variants have associations with an increased risk of obesity. Numerous studies have replicated this finding.[ref][ref][ref][ref][ref][ref]
FTO acts on gene expression of genes involved in fat storage and also increases the number of adipocytes (fat cells) that form. Higher FTO levels, such as from FTO genetic variants, increase both fat storage and the number of fat cells.[ref]
Related article: FTO and weight loss
Diabetes:
One big complication of diabetes is the effect on the heart and vascular system. Even when blood glucose levels are controlled, diabetic vascular complications due to endothelial dysfunction are a problem.
The fact that this happens even when people with diabetes have blood glucose levels under control points to a cause other than high glucose levels.
Researchers have found that vascular endothelial dysfunction is caused by enhanced FTO levels and reduced m6A levels. The flip side is also true – reducing FTO levels in endothelial cells decreased inflammation. Diabetic mice with FTO levels decreased had fewer vascular complications.[ref]
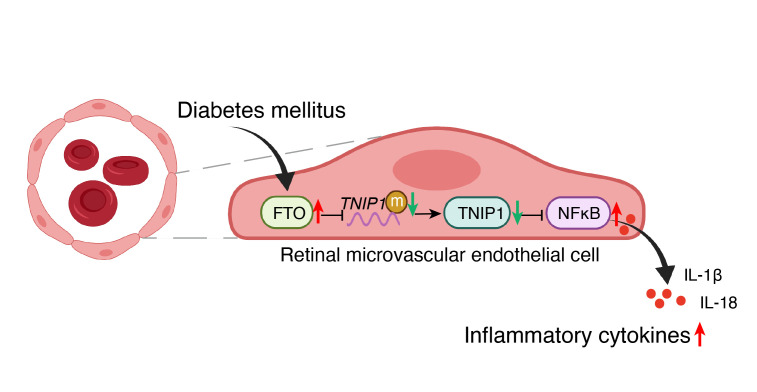
Genetic variants in the FTO gene that are linked to an increased risk of obesity are also linked to an increased risk of diabetes. Researchers have discovered that the increase in type 2 diabetes risk is independent of BMI. This implies that the increase in the risk of diabetes is not due to increasing obesity and is, instead, due to increased FTO.[ref][ref]
Autoimmune diseases:
Obesity is considered a risk factor for many autoimmune diseases with the assumption that overeating or an abundance of adipose tissue is driving the immune system changes.
m6A methylation has been shown in numerous studies to be involved in the pathogenesis of autoimmune diseases through its regulation of antiviral immunity, inflammation, adaptive immune response, and mitochondrial energy metabolism.[ref]
Research shows that aberrant m6A methylation is implicated in the biological process of rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, psoriasis, type 1 diabetes, and inflammatory bowel diseases.[ref][ref]
Neurodevelopmental and Mood Disorders:
Gene expression in the brain and CNS is also affected by m6A methylation during the development of the fetus and then throughout life. FTO plays essential roles in brain development. Low FTO during infancy leads to altered BDNF pathways and cell death in neurons. Low FTO also affects axon growth and synaptic transmission.[ref]
In major depressive disorder, FTO is downregulated in the hippocampus. (This makes me think of the term ‘fat and happy’… higher FTO, not depressed?)
Higher FTO levels in children are associated with a decreased risk of ADHD.[ref][ref]
FTO and m6A in aging:
I’ve focused a lot on the negative aspects of excess FTO or aberrant m6A methylation. There are always positives to genetic variants, including the FTO variants that increase weight.
Here are a couple of examples of how increased FTO is of benefit in aging.
- FTO protects against certain aspects of ovarian aging.[ref]
- The genetic variants that increase FTO (and increase the risk of obesity) protect against muscle loss and sarcopenia in the elderly.[ref]
- The ‘obesity paradox’ refers to the data showing that elderly people who are overweight are more likely to survive. Researchers have a bunch of different hypotheses of why being overweight is a benefit, but there could be tradeoffs where more FTO is beneficial in old age?[ref][ref]
m6A Methylation and Endocrine Disruptors:
BPA and BPS are components of plastic that are ubiquitous in the environment. These endocrine disruptors also interact with m6A methylation by increasing m6A methylation and downregulating FTO in the testes.[ref][ref][ref] In the ovaries, BPA alters m6A methylation causing some genes to be upregulated and others to be downregulated.[ref]
Phthalates, another ubiquitous endocrine disruptor, also interact with m6A methylation. Similarly to bisphenols, some studies show that phthalates downregulate FTO which causes higher m6A methylation.[ref]
The studies on endocrine disruptors and their effect on FTO and m6A methylation are really new, and more research is needed to understand the effect in different tissues.
FTO as the cause, obesity as a bystander?
Does FTO research negate obesity as Causal in chronic diseases?
The FTO genetic variants that increase the amount of FTO produced are strongly linked to an increased BMI. It is the strongest common genetic risk factor.
Higher BMI or obesity is associated in epidemiological studies with a number of comorbidities, including cancer, diabetes, heart disease, autoimmune diseases, and neurodegenerative diseases.
Conventional thinking is that obesity causes cancer, increases susceptibility to illnesses, is a factor in neurodegeneration, and causes autoimmune diseases:
- The American Cancer Society claims that up to 20% of cancer deaths are due to obesity. Being overweight or obese is considered to be a cause of some cancers. Causal instead of correlation – e.g., you ate too much and now have cancer because of it. [ref]
- Being overweight or obese is correlated with being more vulnerable to viral pathogens. The message from some public health people is that you eat too much — and are therefore unhealthy and get sick more often.
- Being overweight or obese is correlated with an increased risk of autoimmune diseases. Does increased fat mass cause your immune system to go haywire in autoimmune diseases? Or, as several studies show, does the aberrant m6A methylation cause the changes to your adaptive immune response?[ref][ref]
Again, the FTO variants are the strongest genetic connection to weight gain. Is the link between obesity and cancer, infectious diseases, and autoimmune diseases that people who are overweight are much more likely to carry the FTO genetic variant that increases FTO? Thus, increased FTO is the upstream cause of all these conditions.
Many studies that are looking at genetic causes use statistical methods to take out the impact of comorbidities, such as higher BMI. In other words, are studies overlooking the impact of the FTO variants due to mitigating BMI? If FTO genetic variants are the ’cause’ rather than obesity, researchers are factoring the FTO variant out of the equation by adjusting for BMI.
The other side of the research picture is that Mendelian randomization studies include FTO as a strong causal association between obesity and cancer, heart disease, autoimmune disease, etc. Assuming that FTO is causal for obesity, then is used to infer that obesity is causal for the other diseases.[ref]
Why does it matter if being overweight causes cancer vs. carrying the FTO variant, causing both overweight and cancer?
When it comes to personalizing interventions and targeting treatment, understanding the mechanism is essential.
- There are many reasons for obesity…and not everyone who is obese carries the FTO variants. Thus, if the FTO variant drives cancer risk, perhaps not everyone who is overweight is at an increased risk of cancer. Focusing on weight may not mitigate cancer risk.
- Conversely, not everyone who carries FTO variants is obese, but they may be at an increased risk of specific cancers from the variants. Knowing what to target is essential to getting the intervention right.
- Generalities (e.g., obesity = increased risk of cancer) are fine when researchers are looking at whole population groups. But generalities don’t mean jack when it comes to an individual who wants to prevent a specific cancer.
Thus, if blaming obesity has caused researchers to miss the FTO variants as a risk factor for cancer, immune system, neurodegeneration, and autoimmune disease – well, that could change a lot of the assumptions made in public health.
Let’s take a look at your FTO genetic variants in more detail, and then I’ll get into the ideas on how to mitigate increased risks.
FTO Variants Genotype Report:
Lifehacks:
I need to emphasize again that much of the research on m6A methylation writers and erasers is new. There is still a lot to learn here, and this is a topic to revisit in a few years.
Folate and choline:
If you have too much FTO due to the genetic variants above, does this mean you should balance this ‘eraser’ with more methyl groups available for the ‘writers’? I don’t think research gives us a definitive answer on this yet, but there is some animal research that points to the need for sufficient methyl donors in the diet.
Both folate and choline act as methyl donors in the diet. In the cell, S-adenosyl methionine (SAMe) is the molecule that donates a methyl group for methylation reactions. Sources of methyl groups for the formation of SAMe include folate (via the folate cycle) and choline (as betaine). When SAMe gives up its methyl group, it becomes SAH (S-adenosyl homocysteine). Higher levels of SAH then feed back to inhibit the m6A writer METTL3.[ref]
An animal study showed that lead toxicity, which causes learning and memory problems, affects m6A methylation by increasing FTO. Interestingly, increasing folate could mitigate part of the learning/memory problems due to lead. The researchers concluded that this was due to folate’s effect on m6A mRNA methylation.[ref]
Related article: Lead detoxification genes
Artimenisen:
A study shows that dihydroartemisinin, derived from the herbal supplement Artimenisen, can alleviate vascular inflammation specifically by downregulation of FTO.[ref]
Curcumin:
Curcumin has been shown in animal studies to increase m6A methylation and affect FTO expression.[ref] Other studies show that curcumin reduces obesity by altering m6A methylation by affecting the other m6A eraser, ALKBH5.[ref]
Eggplant and Ethiopian eggplant:
Researchers have found that eggplant and Ethiopian eggplant decrease FTO expression in animals.[ref]
Green tea:
EGCG, the polyphenol found in green tea, reduces FTO and reduces the formation of fat cells in cell studies.[ref]
Alpha-ketoglutarate and FTO:
Related Article and Topics: