Key takeaways
~ Glutamate is the most abundant neurotransmitter in the brain and periphery.
~ Cells can make glutamate from glutamine or alpha-ketoglutarate.
~ There are genetic variants that impact glutamate levels a bit, but overall, glutamate levels are tightly controlled by multiple pathways.
~ Altered glutamate signaling is implicated in schizophrenia, OCD, and migraines.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
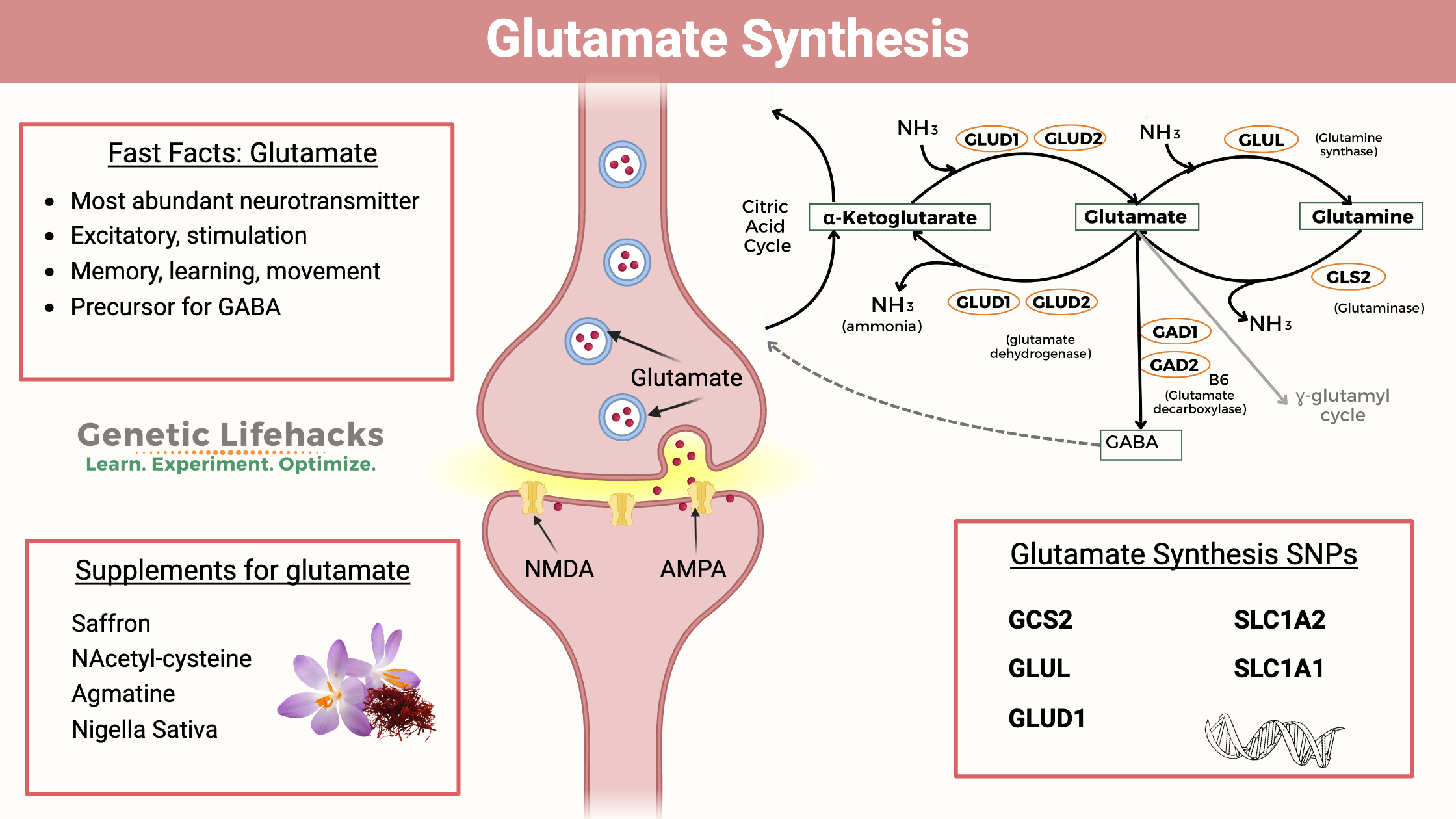
What is glutamate?
Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS). It’s important for learning, memory, and mood, but it’s not as well known as other neurotransmitters like dopamine or serotonin.
As an excitatory neurotransmitter, glutamate is essential for learning, attention, and focus – but too much glutamate causes too much stimulation in the brain. A balance between stimulation and inhibition is needed.
Let’s look at the research on how glutamate is synthesized, what receptors it binds to, and why it is so important for cognitive function.
How glutamate is synthesized:
Glutamate is the most abundant free amino acid in the brain.[ref] As an excitatory amino acid, glutamate levels are strictly controlled by several mechanisms. Glutamate can be synthesized from multiple sources and can also be converted into other neurotransmitters or amino acids. These pathways interact to keep glutamate levels at the right balance.
Here’s a graphical overview of the synthesis pathways:
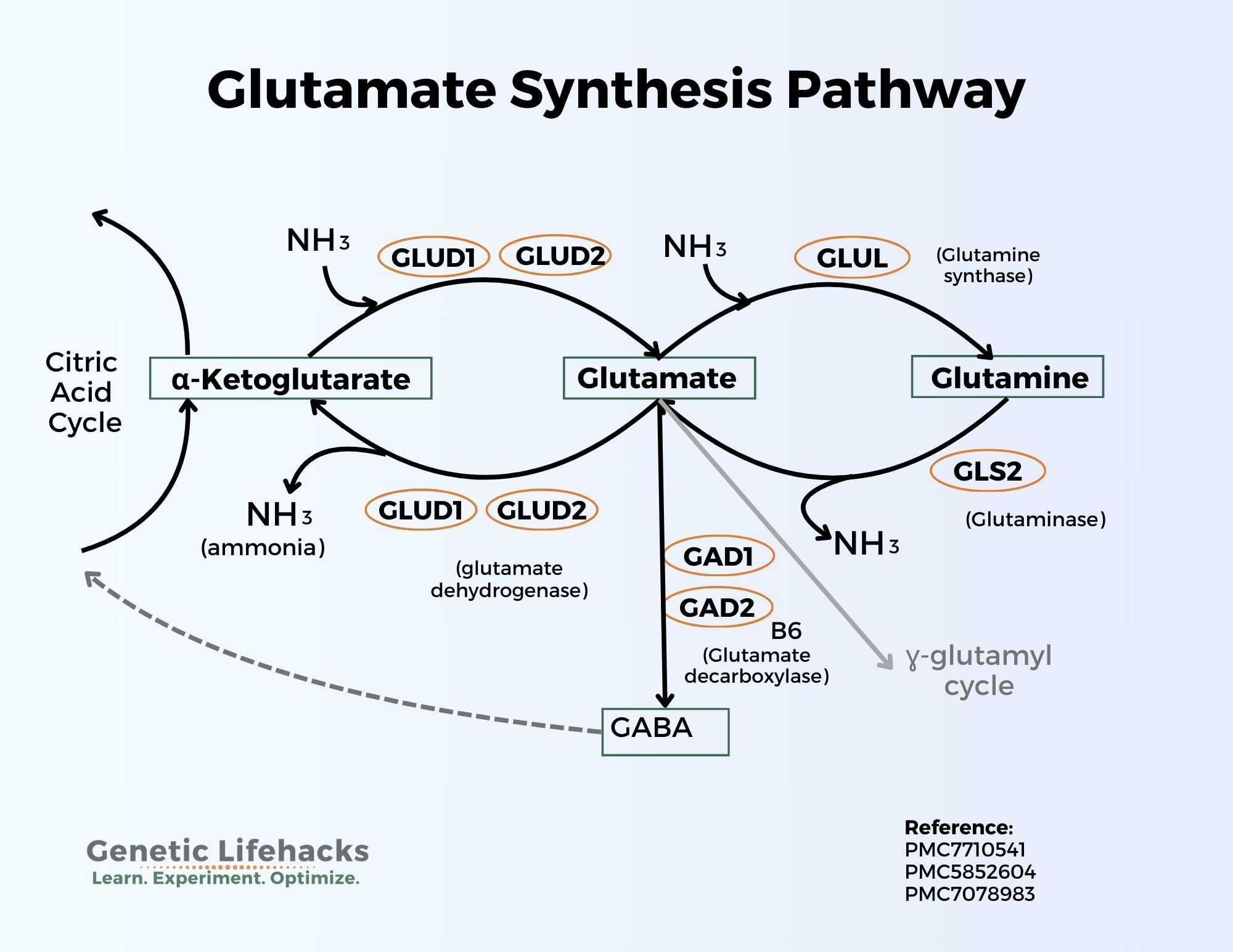
Glutamate from glutamine:
Glutamate can be synthesized from the amino acid glutamine with the help of the enzyme glutaminase (GLS and GLS2 genes). This conversion releases a molecule of ammonia (NH3). In neurons, glutamate is then packaged into synaptic vesicles by vesicular glutamate transporters (VGLUTs) and stored in the presynaptic terminal before release.
The conversion of glutamine to glutamate is a two-way street. Glutamate can also be converted to glutamine with the addition of a molecule of ammonia (NH3).
Glutamate + ATP + NH3 → Glutamine + ADP + phosphate
Glutamine is the most abundant free amino acid in the body. In addition to being used to synthesize glutamate, glutamine is incorporated into many proteins and can be used for nucleotide synthesis. It is considered “conditionally essential,” meaning that most of the time the body can make enough glutamine, but during times of stress (illness, etc.) the demand for glutamine may be such that it is needed from food or supplements.[ref]
Access this content:
An active subscription is required to access this content.