Key takeaways:
~ Glutathione is produced in your cells and acts as a potent antioxidant, protecting cells from oxidative damage.
~ It also supports detoxification, mitochondrial function, and immune system regulation.
~ Genetic variants can impact how well you produce glutathione and detoxify certain toxicants.
~ Natural supplements are available that provide the building blocks for glutathione if you are deficient.
What is glutathione, and what does it do in the body?
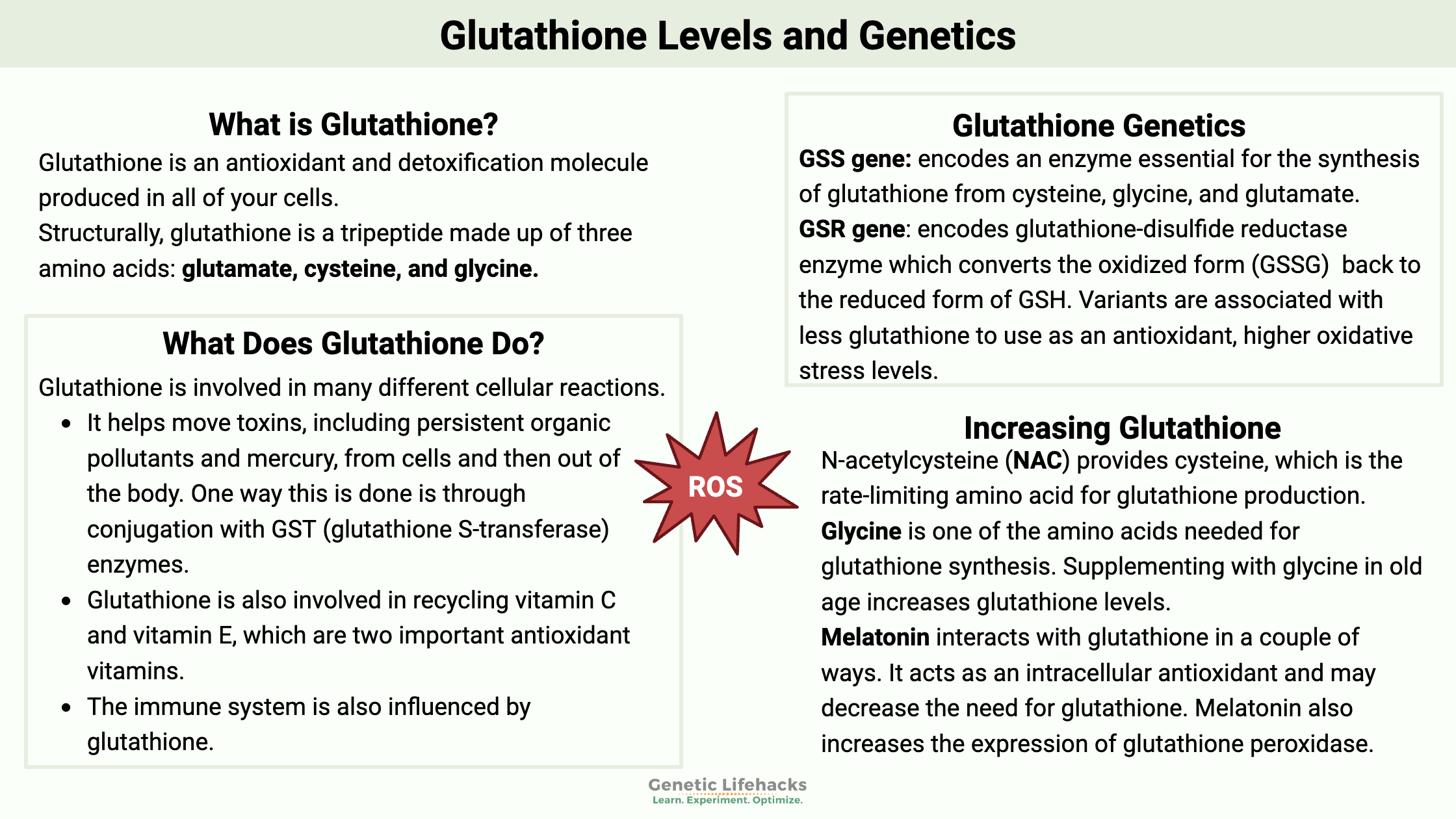
Glutathione is an antioxidant and detoxification molecule that is produced in all of your cells. Structurally, glutathione is a tripeptide made up of three amino acids: glutamate, cysteine, and glycine.
Glutathione exists in two forms as free glutathione:
- the reduced thiol form, which is abbreviated GSH
- the oxidized disulfide form, which is abbreviated GSSG
A thiol is a sulfur compound that contains a sulfhydryl group (-SH), which is a sulfur atom attached to a hydrogen atom.
Under normal, healthy conditions, 98% of free glutathione is in the reduced form (GSH) and is found ubiquitously throughout the body.[ref] The ratio of the reduced form (GSH) to the oxidized form (GSSG) gives us the redox status of the cell. The ratio of reduced to oxidized is over 100 when cells are healthy and at rest. This ratio drops tremendously when cells are under oxidative stress.[ref]
What does glutathione do?
- Glutathione acts as a direct antioxidant. The thiol group is important – this sulfur compound allows glutathione to donate electrons to neutralize reactive oxygen species (ROS) in the cell. In healthy cells, there is a balance between ROS and antioxidant defenses. With excess ROS, cells experience oxidative stress, which is at the root of many chronic diseases.[ref]
- Glutathione can also bind to molecules with the help of detoxification enzymes to eliminate certain types of toxins and heavy metals.
- Another role of glutathione is to modulate the immune system by regulating Treg cells functionality.[ref]
- Glutathione balances the redox state of a cell.
| Function | Description |
|---|---|
| Antioxidant | Neutralizes reactive oxygen species (ROS) and nitrogen species to prevent oxidative stress |
| Detoxification | Binds and eliminates toxins, heavy metals (mercury, arsenic), and persistent organic pollutants |
| Immune Modulation | Regulates Treg cell function and immune system balance |
| Redox Status Maintenance | Maintains cellular redox balance (GSH:GSSG ratio >100 in healthy cells) |
| Vitamin Recycling | Assists in recycling Vitamin C and E |
| Estrogen Metabolism | Involved in breaking down estrogen |
Let’s take a look at these functions in detail…
Glutathione pathways:
Glutathione is involved in many different cellular reactions.[ref][ref]
- It can directly scavenge reactive oxygen species, including hydrogen peroxide, hydroxyl radical, lipid peroxyl radical, superoxide anion, and peroxynitrite.
- It helps move toxins, including persistent organic pollutants and mercury, from cells and then out of the body. One way this is done is through conjugation with GST (glutathione S-transferase) enzymes.
- Glutathione is also involved in the recycling of vitamin C and vitamin E, two important antioxidant vitamins.
- The immune system is also affected by glutathione, with Treg cells helping to prevent autoimmune diseases.[ref]
- One pathway of estrogen metabolism involves glutathione[[ref]
Here’s a specific detox example:
Mercury is a toxic heavy metal that is eliminated from the body in a glutathione-dependent manner. Mercury is conjugated to glutathione by the GST enzymes (GSTT1, GSTM1, or GSTP1). Without sufficient glutathione, mercury can accumulate in the body.ref]
Not the only player in the detox game:
Note that there is a lot of redundancy in cellular processes. Glutathione is an essential antioxidant that interacts with many different processes. However, there are multiple ways that cells can balance ROS and combat oxidative stress. There are also multiple detoxification pathways for many of the toxins that glutathione helps remove. So while glutathione is essential and important, there’s also a lot of redundancy in these systems with multiple ways to keep cells healthy.[ref]
How is glutathione synthesized?
| Enzyme/Gene | Role in Glutathione |
|---|---|
| GCL (Glutamate-Cysteine Ligase) | Synthesis: Rate-limiting step for glutathione synthesis |
| GSR (Glutathione Reductase) | Conversion: Reduces GSSG to GSH using NADPH |
| GGT1 (Gamma-Glutamyltransferase) | Recycles cysteine from conjugated glutathione |
| GPX1 (Glutathione Peroxidase) | Neutralizes hydrogen peroxide, lipid peroxides |
| GSTT1, GSTM1, GSTP1 (GST Family) | Detoxifies heavy metals and persistent pollutants using glutathione |
Let’s look at each of these, and then you can check your genes below in the genotype report section.
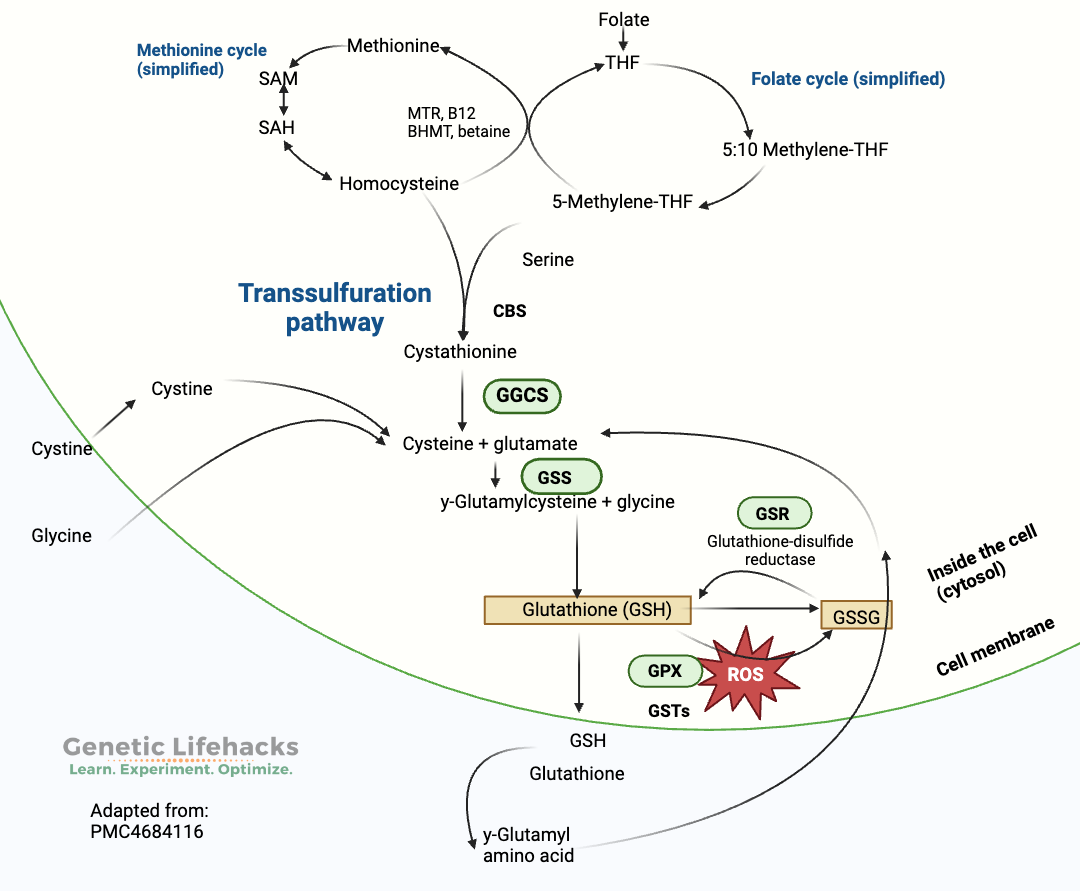
Glutathione is synthesized from glycine, cysteine, and glutamate in a two-step process that occurs in the cytosol of all cells. It is a tightly regulated reaction that depends on both the availability of the amino acids (cysteine, glycine, and glutamate) and the rate-limiting enzyme, glutamate-cysteine ligase (GCL). [ref]
- The glutamate-cysteine ligase (GCL) enzyme interacts with glutamate and cysteine, resulting in the formation of gamma-glutamylcysteine.
- In the second step, glycine is added to the gamma-glutamylcysteine using the enzyme glutathione synthetase to form glutathione.
While all cells can synthesize glutathione for use within the cell, the liver also produces glutathione in larger amounts and exports it into the plasma and bile. [ref]
Related article: Glutamate regulation
Regulation of synthesis:
The amount of the amino acid cysteine in the cell is the limiting factor for glutathione production in a cell. Cysteine is an amino acid that we get from foods, such as meat, eggs, and lentils, and it can also be synthesized in the body from methionine through the transsulfuration pathway.[ref]
Recycling and Re-oxidation:
Glutathione doesn’t need to be constantly made in cells. When glutathione is involved in antioxidant reactions, it becomes the oxidized form, GSSG (glutathione disulfide). The oxidized form, GSSG, can be reduced back to reduced glutathione with the help of glutathione reductase (GSR gene) and NADPH. This is a constant process-oxidizing, reducing over and over again[ref]
Another way that glutathione can be formed is by recycling cysteine from conjugated glutathione. This process uses the GGT1 enzyme.[ref][ref]
Enzyme reactions involving glutathione:
Glutathione peroxidase (GPX1 gene, below) is an important enzyme that utilizes glutathione in antioxidant reactions, such as in neutralizing hydrogen peroxide and lipid peroxide. In these antioxidant reactions, glutathione is oxidized to form the oxidized disulfide form, GSSG — which is then, in turn, reduced back to glutathione using NADPH.[ref]
Mitochondria are essential for the production of ATP for energy storage in cells. However, the process of making ATP also produces reactive oxygen species (ROS), such as hydrogen peroxide, which can damage the cell at higher levels. Thus, glutathione is an essential part of the way cells balance ROS on a constant basis – as well as being available in situations where oxidative stress is generated by toxins, disease, or other pathological conditions.[ref]
Glutathione peroxidase (GPX1, GPX2,3,4) — antioxidant in cells
Glutathione S-transferases (GSTs) are a family of enzymes that bind glutathione to toxic substances to remove them from the cell and then out of the body. Binding the toxin to glutathione allows it to be easily moved out of the cell via several different transmembrane efflux pumps. The glutathione-bound toxic substance can then be excreted in the urine, sweat, or feces.[ref]
Glutathione S-transferase (GSTM1, GSTP1, etc) — detoxification reactions
Related article: GST Genetic Variants
What depletes your glutathione levels?
| Factor | Mechanism/Example |
|---|---|
| Environmental Toxins | Mercury, arsenic, gadolinium |
| Autoimmune Diseases | Lupus, RA, Crohn’s, MS, and psoriasis increase oxidative stress |
| Cystic Fibrosis | Lung inflammation depletes glutathione |
| Alcohol Use | Metabolism produces acetaldehyde, leading to depletion |
| Mold/Mycotoxins | Glutathione conjugates and removes mycotoxins |
| Acetaminophen Usage | Detoxifies NAPQI, depletion risk with long-term use, alcohol |
Environmental toxin exposure:
Glutathione is the primary intracellular antioxidant and is therefore important for preventing oxidative stress in cells due to environmental toxin exposure. Here are three examples:
- Exposure to gadolinium in contrast agents increases oxidative stress. Animal studies show that gadolinium can cause low glutathione levels.[ref]
- Mercury is another heavy metal that utilizes glutathione in the detoxification pathways.[ref]
- Arsenic exposure causes increased glutathione usage. Glutathione can interact with arsenic to reduce it (redox reaction), and glutathione also can bind with arsenic, causing higher glutathione excretion through bile. A 2013 study in Bangladesh looked at people exposed to different levels of arsenic in their drinking water. Those who were at higher levels of arsenic exposure had lower glutathione levels. [ref]
Related articles: Mercury detox genes & Arsenic detox genes
Autoimmune diseases:
Glutathione plays a role in neutralizing oxidative stress in autoimmune diseases, and it also modulates the immune response by balancing the Th1/Th2 response. Low glutathione levels are found in people with lupus, rheumatoid arthritis (RA), Crohn’s disease (IBD), multiple sclerosis (MS), and psoriasis.[ref]
Related articles: Lupus, Rheumatoid arthritis, IBD, MS, and psoriasis genes
Cystic fibrosis:
People with cystic fibrosis, which is a genetic disease caused by mutations in the CFTR gene, have frequent respiratory infections that cause lung inflammation and produce oxidative stress in the lungs, which depletes glutathione levels. A number of studies have looked at increasing glutathione levels in people with CF to improve lung function.[ref]
Related article: Cystic fibrosis: How to check to see if you are a carrier of a CFTR mutation
Alcohol:
Long-term or heavy alcohol use depletes glutathione levels in the liver, causing alcoholic liver disease. Studies show that glutathione, or glutathione plus zinc, may help mitigate liver damage from chronic alcohol use. Alcohol is metabolized to acetaldehyde, which is toxic. Administration of glutathione reduces serum acetaldehyde levels.[ref][ref][ref][ref]
Related article: Genes related to alcohol and acetaldehyde metabolism
Mold and mycotoxins:
Mold can produce mycotoxins, which are toxic substances that are harmful to human health. One way that mycotoxins, such as aflatoxin, are removed from the body is by conjugation with glutathione.[ref]
Related article: Mycotoxins and genetic variants related to detoxification
Acetaminophen:
The metabolism of acetaminophen produces a toxic metabolite called NAPQI, which is neutralized and excreted by conjugation with glutathione. When higher levels of acetaminophen are ingested, the liver’s supply of glutathione is depleted and NAPQI causes liver damage.[ref]
Related article: Genetic variants that increase the risk of liver damage from acetaminophen
Studies on the impacts of low glutathione levels:
Glutathione levels in the body are fairly tightly regulated, and balance is key. We need enough available to prevent excess ROS from causing oxidative stress and damage to cells. However, the upregulation of glutathione can be a negative factor when it comes to preventing out-of-control cellular growth in cancer.[ref]
Low glutathione levels are found in a number of different chronic diseases. However, there aren’t many studies showing that low glutathione directly causes chronic disease. Instead, it may be that chronic diseases cause low glutathione levels, which then can exacerbate the conditions due to an inability to counteract the increasing levels of oxidative stress.
- Type 2 diabetes:
Oxidative stress plays a role in diabetes, and people with type 2 diabetes have lower glutathione levels on average.[ref] - Pancreatitis:
Chronic pancreatitis causes oxidative stress. Patients with chronic pancreatitis have lower levels of glutathione, cysteine, and glycine.[ref] Increasing cysteine levels with NAC is protective against pancreatic damage.[ref] - Schizophrenia:
Several research studies show that people with schizophrenia have lower glutathione levels in certain regions of the brain.[ref] - In aging:
The studies on glutathione in aging show that there is usually a general decrease in serum glutathione levels as we age. An increase in oxidative stress and cell damage causes an increased usage of glutathione and decreased levels. However, brain glutathione levels may be relatively stable or regionally affected. This lack of clear association between brain glutathione levels and aging is important because it shows that glutathione may not be the limiting factor in cognitive changes in aging. Instead, low glutathione in the rest of the body could be due to an increased use of it for combating oxidative stress.[ref]
Age spots on the skin:
Melanin is what gives skin its color, with eumelanin being darker than pheomelanin. In aging, dark spots on the skin may be caused by free radicals that cause eumelanin formation.[ref] Glutathione has been shown in a number of studies to lighten age spots, and it is thought that both its antioxidant effect and a shift towards pheomelanin formation are at the root of these antiaging skin properties.[ref]
Let’s switch gears here and look at how your genetic variants impact your need for glutathione:
Genotype report:
Access this content:
An active subscription is required to access this content.
Lifehacks:
If you think you may need to boost your glutathione levels due to oxidative stress and genetic polymorphisms, there are multiple way you could approach this:
- Reduce the need for glutathione (reduce toxins)
- Add more cysteine — it’s the limiting factor for glutathione synthesis
- Also, add glycine if it is limited in your diet
- Directly supplement with liposomal or IV glutathione
- Indirectly supplement with cofactors
Avoiding toxicants:
Avoiding exposure to the toxicants that deplete glutathione will increase your overall glutathione levels and give you more resistance to oxidative stress. Here are three of the big ones to avoid.
Arsenic:
One reason we need glutathione is to detoxify arsenic. Arsenic occurs naturally in the soil in some areas of the world and can leach into well water. If you live in an area where arsenic is common in the water, be sure to have your water tested. Arsenic is also absorbed by rice, especially the rice bran found in brown rice.
Related article: Arsenic, detoxification, and your genes
Mercury:
Methylmercury is the type of organic mercury we get from eating mercury-containing fish. Glutathione conjugates with methylmercury for elimination. Mercury is stored in the brain at higher levels than in the rest of the body. Without enough glutathione, mercury can damage the brain and also take the place of selenium in certain biological pathways.[ref]
Related article: Mercury, detoxification, and your genes
Alcohol:
Acute or chronic alcohol exposure leads to a depletion of glutathione in the liver and liver damage. Glutathione levels also decline with age and with chronic diseases, so in either situation, the ability of the liver to detoxify alcohol and recover from alcohol intake is diminished due to a lack of glutathione.[ref]
N-acetylcysteine (NAC):
Cysteine isn’t available as a stand-alone supplement because it is unstable and easily oxidized. Instead, NAC is used to provide cysteine in a stable supplement form.
N-acetylcysteine, or NAC, is a supplement that works in two ways:
- It provides cysteine, which is the rate-limiting amino acid for glutathione production
- It can also act as a direct antioxidant to scavenge ROS (NO2 and HOX)[ref][ref]
The main antioxidant mechanism for NAC is by increasing glutathione levels. However, the direct antioxidant activity is through breaking disulfide bonds, which is what it does in the lungs to break up mucus. NAC has long been used as a mucolytic agent — it thins out mucus — for COPD patients.[ref][ref]
Genetic connection: GGT1 gene
Studies show that NAC decreases GGT levels, indicating that it increases the overall supply of glutathione and reduces the need to recycle it.[ref] For people with GGT1 genetic variants, this may make NAC particularly helpful.
What’s the downside of NAC?
While generally safe at supplemental doses, a drawback to N-acetyl cysteine is the sulfur smell and taste. This is definitely one supplement to take in capsule form instead of as a powder. The other drawback is that some people who are sensitive to higher histamine levels or who have mast cell activation syndrome report that NAC gives them a histamine reaction.
In addition, increasing NAC and glutathione could be detrimental if you are undergoing therapy for certain types of cancer because some tumor cells thrive with more glutathione.[ref] Always talk with your doctor if you are undergoing treatment or have medical questions.
Dosage of NAC:
Studies of NAC for many different conditions have used doses ranging from 500 mg to up to 20 g/day. A meta-analysis of studies using NAC to increase athletic performance found that there were more side effects (none severe) at 20g/day.[ref] High doses of NAC are used in hospitals by IV for reversing acetaminophen toxicity.
Here are some clinical trials to give you an idea of the range of dosages:
- For schizophrenia, a clinical trial using 2400 mg/day of NAC showed increased glutathione levels and decreased glutamate levels. However, it didn’t cure schizophrenia.[ref]
- For COPD, a meta-analysis showed that lower doses (1200mg/day or less) for a duration of three months seemed to work best.[ref]
- A study in MS patients showed that 600 mg/2x/day did not significantly increase serum glutathione levels. However, it did improve anxiety symptoms in the patients.[ref]
- A meta-analysis of clinical trials using 900 – 2700 mg/day showed that NAC may improve hearing loss (noise-induced).[ref]
Keep in mind that these studies involved patients who were likely to have increased glutathione use. If you don’t have a chronic condition, your need for NAC, cysteine, or glutathione may be less.
Safety and side effects:
A meta-analysis of multiple studies in COPD patients showed no significant adverse effects at doses ranging from 600 mg to 3g/day.[ref] Studies in healthy adult athletes go up to 20g/day (not recommending this, just including it for safety).[ref] Conversely, in Parkinson’s patients, there was a high number of adverse events at 6g/day of NAC. [ref]
Talk with your doctor or pharmacist if you are on prescription medications to see if there is any interaction with any new supplement before you start it.
If you have cancer:
In cancer, cysteine and glutathione may help tumor cells resist some types of chemotherapy.[ref] Talk to your oncologist or doctor about whether NAC or anything that boosts glutathione is a good idea for you. There are multiple types of cancer, and NAC may be beneficial in some while harmful in others.
Side effects:
Some people report histamine-related side effects from NAC. An animal study showed that NAC increased histamine release in conjunction with fluoride.[ref] In people given IV n-acetylcysteine in a hospital setting for acetaminophen poisoning, about 8% of the patients had an “anaphylactoid reaction, defined as cutaneous (urticaria, pruritus, angioedema) or systemic (hypotension, respiratory symptoms)” within five hours of receiving the IV. Most were skin-related symptoms, but anaphylaxis is possible with IV NAC.[ref]
Some people also report gastrointestinal side effects with NAC. Clinical trials show that gastrointestinal side effects are at a similar rate to the placebo group.
Directly supplementing with glutathione:
Access this content:
An active subscription is required to access this content.
Related articles:
Pyruvate Dehydrogenase Deficiency – Mitochondrial Dysfunction and Lactate Production
Bibliography
Aldini, Giancarlo, et al. “N-Acetylcysteine as an Antioxidant and Disulphide Breaking Agent: The Reasons Why.” Free Radical Research, vol. 52, no. 7, July 2018, pp. 751–62. PubMed, https://doi.org/10.1080/10715762.2018.1468564.
Andreoli, Virginia, and Francesca Sprovieri. “Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview.” International Journal of Environmental Research and Public Health, vol. 14, no. 1, Jan. 2017, p. 93. pmc.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijerph14010093.
Atkuri, Kondala R., et al. “N-Acetylcysteine – a Safe Antidote for Cysteine/Glutathione Deficiency.” Current Opinion in Pharmacology, vol. 7, no. 4, June 2007, p. 355. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.coph.2007.04.005.
Awuchi, Chinaza Godseill, et al. “Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review.” Toxins, vol. 14, no. 3, Feb. 2022, p. 167. pmc.ncbi.nlm.nih.gov, https://doi.org/10.3390/toxins14030167.
Ballatori, Nazzareno, et al. “Glutathione Dysregulation and the Etiology and Progression of Human Diseases.” Biological Chemistry, vol. 390, no. 3, Mar. 2009, p. 191. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1515/BC.2009.033.
Bray, George A., et al. “Is There an Ideal Diet? Some Insights from the POUNDS Lost Study.” Nutrients, vol. 16, no. 14, July 2024, p. 2358. PubMed Central, https://doi.org/10.3390/nu16142358.
Calverley, Peter, et al. “Safety of N-Acetylcysteine at High Doses in Chronic Respiratory Diseases: A Review.” Drug Safety, vol. 44, no. 3, Dec. 2020, p. 273. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1007/s40264-020-01026-y.
Chang, Po-Hsiung, et al. “Effect of N-Acetyl-Cysteine in Prevention of Noise-Induced Hearing Loss: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.” Archives of Medical Science : AMS, vol. 18, no. 6, Mar. 2021, p. 1535. pmc.ncbi.nlm.nih.gov, https://doi.org/10.5114/aoms/109126.
Ciofu, Oana, et al. “Antioxidant Supplementation for Lung Disease in Cystic Fibrosis.” Cochrane Database of Systematic Reviews, no. 10, 2019. www.cochranelibrary.com, https://doi.org/10.1002/14651858.CD007020.pub4.
Coimbra, Susana, et al. “Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents—A Review.” International Journal of Molecular Sciences, vol. 25, no. 7, Apr. 2024, p. 4071. pmc.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms25074071.
Coles, Lisa D., et al. “Repeated-Dose Oral N-Acetylcysteine in Parkinson’s Disease: Pharmacokinetics and Effect on Brain Glutathione and Oxidative Stress.” Journal of Clinical Pharmacology, vol. 58, no. 2, Feb. 2018, pp. 158–67. PubMed, https://doi.org/10.1002/jcph.1008.
Deponte, Marcel. “The Incomplete Glutathione Puzzle: Just Guessing at Numbers and Figures?” Antioxidants & Redox Signaling, vol. 27, no. 15, Nov. 2017, p. 1130. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1089/ars.2017.7123.
Detcheverry, Flavie, et al. “Changes in Levels of the Antioxidant Glutathione in Brain and Blood across the Age Span of Healthy Adults: A Systematic Review.” NeuroImage. Clinical, vol. 40, 2023, p. 103503. PubMed, https://doi.org/10.1016/j.nicl.2023.103503.
Fang, Yun-Zhong, et al. “Free Radicals, Antioxidants, and Nutrition.” Nutrition, vol. 18, no. 10, Oct. 2002, pp. 872–79. ScienceDirect, https://doi.org/10.1016/S0899-9007(02)00916-4.
Feng, Yinrui, et al. “Zinc-Glutathione Mitigates Alcohol-Induced Intestinal and Hepatic Injury by Modulating Intestinal Zinc-Transporters in Mice.” The Journal of Nutritional Biochemistry, vol. 132, Oct. 2024, p. 109697. PubMed, https://doi.org/10.1016/j.jnutbio.2024.109697.
Goodrich, Jaclyn M., et al. “Glutathione Enzyme and Selenoprotein Polymorphisms Associate with Mercury Biomarker Levels in Michigan Dental Professionals.” Toxicology and Applied Pharmacology, vol. 257, no. 2, Sept. 2011, p. 301. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.taap.2011.09.014.
Hall, Megan N., et al. “Chronic Arsenic Exposure and Blood Glutathione and Glutathione Disulfide Concentrations in Bangladeshi Adults.” Environmental Health Perspectives, vol. 121, no. 9, Sept. 2013, pp. 1068–74. DOI.org (Crossref), https://doi.org/10.1289/ehp.1205727.
Jaeschke, Hartmut, et al. “Novel Therapeutic Approaches Against Acetaminophen-Induced Liver Injury and Acute Liver Failure.” Toxicological Sciences, vol. 174, no. 2, Jan. 2020, p. 159. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1093/toxsci/kfaa002.
Khalatbari Mohseni, Golsa, et al. “Effects of N-Acetylcysteine on Oxidative Stress Biomarkers, Depression, and Anxiety Symptoms in Patients with Multiple Sclerosis.” Neuropsychopharmacology Reports, vol. 43, no. 3, Sept. 2023, pp. 382–90. PubMed, https://doi.org/10.1002/npr2.12360.
Lu, Shelly C. “GLUTATHIONE SYNTHESIS.” Biochimica et Biophysica Acta, vol. 1830, no. 5, Sept. 2012, p. 3143. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.bbagen.2012.09.008.
—. “REGULATION OF GLUTATHIONE SYNTHESIS.” Molecular Aspects of Medicine, vol. 30, no. 1–2, June 2008, p. 42. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.mam.2008.05.005.
Minati, Marie-Albane, et al. “N-Acetylcysteine Reduces the Pro-Oxidant and Inflammatory Responses during Pancreatitis and Pancreas Tumorigenesis.” Antioxidants, vol. 10, no. 7, July 2021, p. 1107. www.mdpi.com, https://doi.org/10.3390/antiox10071107.
Mitrić, Aleksandra, and Immacolata Castellano. “Targeting Gamma-Glutamyl Transpeptidase: A Pleiotropic Enzyme Involved in Glutathione Metabolism and in the Control of Redox Homeostasis.” Free Radical Biology & Medicine, vol. 208, Nov. 2023, pp. 672–83. PubMed, https://doi.org/10.1016/j.freeradbiomed.2023.09.020.
Pizzorno, Joseph. “Glutathione!” Integrative Medicine: A Clinician’s Journal, vol. 13, no. 1, Feb. 2014, p. 8. pmc.ncbi.nlm.nih.gov, https://pmc.ncbi.nlm.nih.gov/articles/PMC4684116/.
Rhodes, Kate, and Andrea Braakhuis. “Performance and Side Effects of Supplementation with N-Acetylcysteine: A Systematic Review and Meta-Analysis.” Sports Medicine (Auckland, N.Z.), vol. 47, no. 8, Aug. 2017, pp. 1619–36. PubMed, https://doi.org/10.1007/s40279-017-0677-3.
Song, Byoung-Joon, et al. “Mitochondrial Dysfunction and Tissue Injury by Alcohol, High Fat, Nonalcoholic Substances and Pathological Conditions through Post-Translational Protein Modifications.” Redox Biology, vol. 3, Oct. 2014, p. 109. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.redox.2014.10.004.
Song, Gunju, et al. “Effects of GSH on Alcohol Metabolism and Hangover Improvement in Humans: A Randomized Double-Blind Placebo-Controlled Crossover Clinical Trial.” Nutrients, vol. 16, no. 19, Sept. 2024, p. 3262. pmc.ncbi.nlm.nih.gov, https://doi.org/10.3390/nu16193262.
Starek-Świechowicz, Beata, et al. “Endogenous Estrogens—Breast Cancer and Chemoprevention.” Pharmacological Reports, vol. 73, no. 6, Aug. 2021, p. 1497. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1007/s43440-021-00317-0.
Vašková, Janka, et al. “Glutathione-Related Enzymes and Proteins: A Review.” Molecules, vol. 28, no. 3, Feb. 2023, p. 1447. pmc.ncbi.nlm.nih.gov, https://doi.org/10.3390/molecules28031447.
Verlaan, Mariette, et al. “Assessment of Oxidative Stress in Chronic Pancreatitis Patients.” World Journal of Gastroenterology : WJG, vol. 12, no. 35, Sept. 2006, p. 5705. pmc.ncbi.nlm.nih.gov, https://doi.org/10.3748/wjg.v12.i35.5705.
Villarama, C. D., and H. I. Maibach. “Glutathione as a Depigmenting Agent: An Overview.” International Journal of Cosmetic Science, vol. 27, no. 3, June 2005, pp. 147–53. PubMed, https://doi.org/10.1111/j.1467-2494.2005.00235.x.
Weschawalit, Sinee, et al. “Glutathione and Its Antiaging and Antimelanogenic Effects.” Clinical, Cosmetic and Investigational Dermatology, vol. 10, Apr. 2017, p. 147. pmc.ncbi.nlm.nih.gov, https://doi.org/10.2147/CCID.S128339.
Wetering, Cheryl van de, et al. “Glutathione S-Transferases and Their Implications in the Lung Diseases Asthma and Chronic Obstructive Pulmonary Disease: Early Life Susceptibility?” Redox Biology, vol. 43, May 2021, p. 101995. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.redox.2021.101995.
Wu, Guoyao, et al. “Glutathione Metabolism and Its Implications for Health.” The Journal of Nutrition, vol. 134, no. 3, Mar. 2004, pp. 489–92. ScienceDirect, https://doi.org/10.1093/jn/134.3.489.
—. “Glutathione Metabolism and Its Implications for Health.” The Journal of Nutrition, vol. 134, no. 3, Mar. 2004, pp. 489–92. ScienceDirect, https://doi.org/10.1093/jn/134.3.489.
Xin, Lijing, et al. “Genetic Polymorphism Associated Prefrontal Glutathione and Its Coupling With Brain Glutamate and Peripheral Redox Status in Early Psychosis.” Schizophrenia Bulletin, vol. 42, no. 5, Apr. 2016, p. 1185. pmc.ncbi.nlm.nih.gov, https://doi.org/10.1093/schbul/sbw038.
Yang, Yvonne S., et al. “N-Acetylcysteine Effects on Glutathione and Glutamate in Schizophrenia: A Preliminary MRS Study.” Psychiatry Research. Neuroimaging, vol. 325, Sept. 2022, p. 111515. PubMed, https://doi.org/10.1016/j.pscychresns.2022.111515.
Zhitkovich, Anatoly. “N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More.” Chemical Research in Toxicology, vol. 32, no. 7, July 2019, pp. 1318–19. PubMed, https://doi.org/10.1021/acs.chemrestox.9b00152.