Key takeaways:
~ The HIF-1a gene encodes a transcription factor that regulates gene transcription in response to oxygen levels, allowing cells to respond to hypoxia (low oxygen).
~ Under low oxygen conditions, HIF-1a increases the transcription of genes involved in oxygen and glucose transport and promotes processes like blood vessel growth and red blood cell production.
~ HIF-1a is often upregulated in cancer and it can increase tumor growth by enhancing blood supply.
~ Genetic variants in the HIF1A gene are linked to athletic performance as well as cancer risk.
HIF-1a background information:
Have you ever wondered, though, how your cells can survive for a few minutes without oxygen — or how your body manages when oxygen levels are lower than normal? It turns out that we have an innate system that detects when oxygen levels are low and turns on other genes that can help cells survive when precious O2 is not readily available.
The hypoxia-inducible factor-1 alpha (HIF1A) gene codes for a transcription factor, which regulates the rate at which certain other genes get transcribed.
So what is a transcription factor, and why is HIF-1a so important?
Genes code for proteins; your cells need to know which genes need to be translated into the proteins needed at that instance. Basically, a transcription factor is like a switch that can turn on or off the transcription of genes. There are a couple of thousand different transcription factors in the human genome.
HIF-1a (hypoxia-inducible factor-1 alpha) responds to the amount of oxygen available to the cell. Hypoxia refers to a state where there isn’t enough oxygen available.
Thus, when there isn’t enough oxygen available at a cellular level, the HIF-1a transcription factor can change the transcription rate for the genes that code for proteins involved in oxygen and glucose transport. It switches on the genes needed for resolving the problems of low oxygen.
When oxygen levels are low (hypoxia), cells and tissues need to respond quickly. The use of oxygen normally occurs in the cellular process to make energy or ATP. (Yes, your cells have a backup route of anaerobic glycolysis, which works without oxygen, but this isn’t as efficient for making ATP.)
Thus, when oxygen levels drop, HIF-1a levels rise, kicking off a bunch of processes.
One way that HIF-1a helps your body respond to the lack of oxygen is by stimulating the growth of more blood vessels. It also stimulates the production of red blood cells by increasing erythropoietin (EPO) expression.
Hypoxia: Altitude, exercise
The first thing that comes to mind with low oxygen levels may be high altitudes. As you go up a mountain in Colorado – or in the Himalayas – oxygen levels decrease. Anyone who has flown out to Colorado to ski at Breckenridge (or worse, A-basin) knows how bad it feels if you don’t take time to adjust slowly to the altitude. (Spend that first night in Denver. Seriously…)
Another way that oxygen levels can drop in your body is during aerobic exercise. You know… like when it is New Year’s Day, and you decided to join the gym and run on the treadmill — for the first time in a year. Your muscles are suddenly using up oxygen faster than you can suck it in, leaving you gasping and feeling like you’re going to die.
On a cellular or tissue level, hypoxia can occur in many situations. First, inflammation can cause hypoxia in a specific area of the body. For example, an inflamed joint due to arthritis will have a low supply of oxygen. Other conditions that cause hypoxia include heart disease, stroke, and kidney disease. HIF-1a levels show increases in all of these chronic inflammatory conditions.[ref][ref][ref]
Hypoxia, HIF-1a, and cancer:
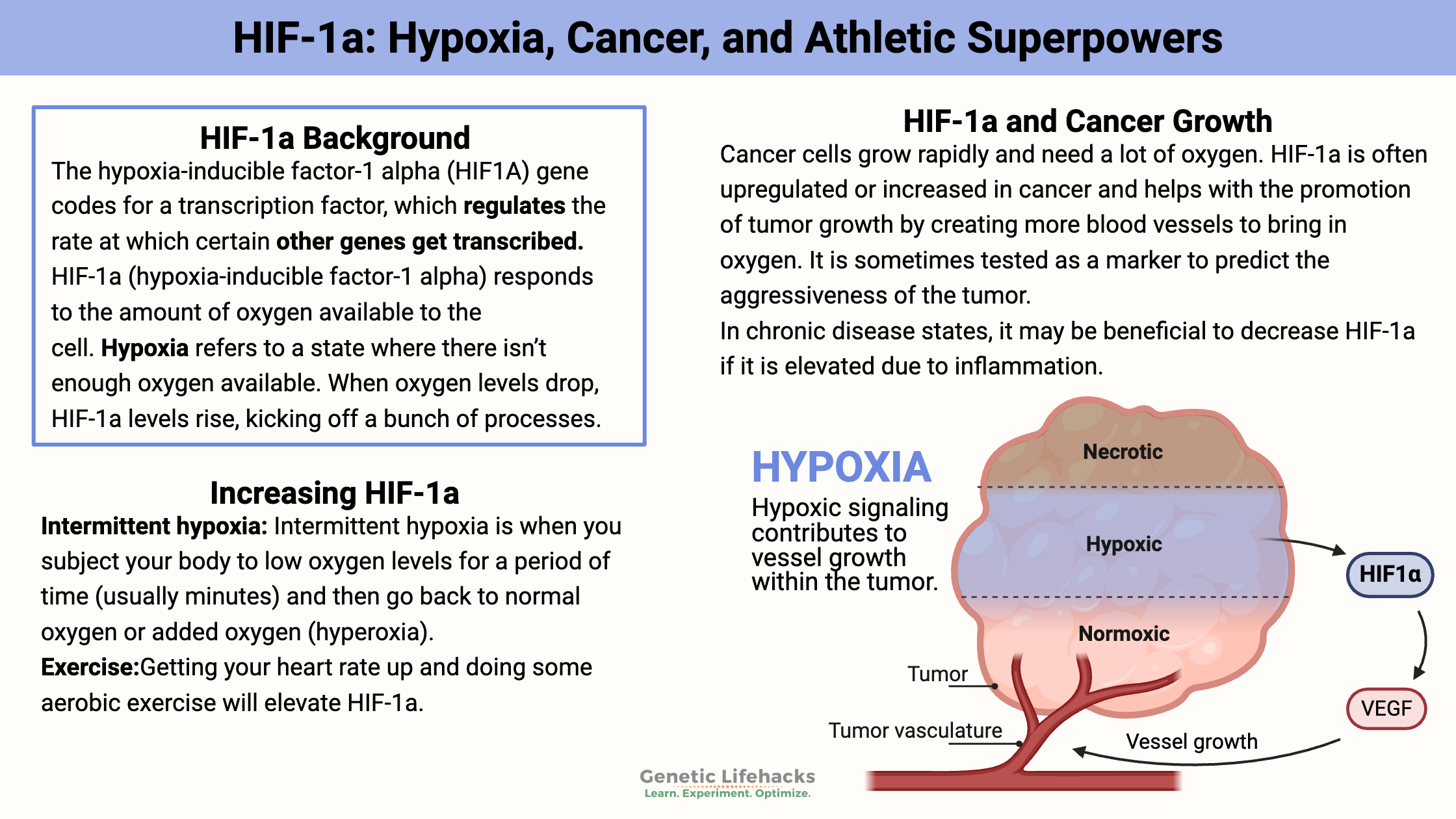
Cancer cells grow rapidly and need a lot of oxygen. Thus, they need more blood vessels to bring in the O2, and they need nutrients.
HIF-1a is very often upregulated or increased in cancer and helps promote tumor growth by creating more blood vessels to bring in oxygen. It is sometimes tested as a marker to predict the aggressiveness of the tumor.
One study showed HIF-1a levels were increased in >90% of colon, lung, and prostate cancers. In addition to causing an increase in blood vessel formation (angiogenesis) to carry oxygen to the tumors, HIF-1a also decreases a cell’s DNA repair mechanism. This increases the rate of mutation in a cell, which is a big problem in tumor cells. Additionally, HIF-1a increases glucose transport, thus providing more energy to cancer cells.[ref][ref]
What does HIF-1a do under normal conditions?
Under normal oxygen levels, known as normoxia, cells produce HIF1a and then quickly degrade it using enzymes called prolyl hydroxylases (PHDs). These PHDs are oxygen sensors, altering HIF-1a and degrading it when there is plenty of oxygen present in the cell.[ref]
Under normal oxygen conditions (normoxia), HIF1A is rapidly degraded. However, in hypoxic conditions, the degradation is inhibited, leading to the accumulation of HIF1A in the cell. This protein then translocates to the nucleus, where it dimerizes with HIF1B and binds to hypoxia-responsive elements (HREs) in the DNA, activating the transcription of various target genes.
What happens if you increase HIF-1a under normal oxygen conditions?
Researchers recently developed ways of inhibiting PHDs, thus allowing HIF-1A to be upregulated under normal oxygen conditions. These PHD (prolyl hydroxylase) inhibitors may be used for regenerating damaged tissue (increased blood vessel formation) or for treating specific types of anemia since increased HIF1A stimulates red blood cell production.
The flip side of encouraging HIF-1a under normoxia is that you really don’t want to encourage cancer growth. As a result, the use of PHD inhibitors only applies to specific conditions.
What else can activate HIF-1a?
In addition to activation by hypoxia, the activation of HIF-1a also occurs from cytokines, growth factors, hormones, and cancer genes. For example, the growth factor IGF-1 can increase HIF1a. Estrogen can also increase HIF-1a, such as in the thickening of the endometrium each month for premenopausal women.[ref][ref][ref]
Interestingly, tamoxifen, a treatment for estrogen-positive breast cancer, works in part by reducing HIF-1a levels.[ref]
Cytokines are inflammatory molecules that the body produces as part of the immune response. They are signals to increase inflammation. Specifically, the cytokine TNF-alpha causes an increase in HIF-1a. [ref] This is one connection between inflammatory conditions, such as arthritis or atherosclerosis, and higher HIF-1a levels.[ref]
HIF1A Genotype Report:
Access this content:
An active subscription is required to access this content.
Lifehacks:
There are natural ways to both inhibit HIF1a and increase HIF1a. I’m listing both here – you can decide which way is best for your body right now.
Logically, if you are trying to increase HIF1a, you may not want to take an inhibitor of HIF1a at the same time. For example, if you are taking resveratrol (inhibitor) while also trying to increase HIF1a through breathwork, you may not reap as much benefit.
Increasing HIF-1a:
Intermittently raising HIF1a may have benefits for your body. Just like exercise causes stress on the body at the moment, the benefits afterward outweigh the short-term stress on the body. Similarly, intermittently raising HIF-1a levels could be of benefit in certain situations.
Intermittent hypoxia:
Just like it sounds, intermittent hypoxia is when you subject your body to low oxygen levels for a period of time (usually minutes) and then go back to either normal oxygen or added oxygen (hyperoxia).
Intermittent hypoxia reduces fasting blood glucose levels and decreases LDL cholesterol in people with prediabetes. The study used middle-aged and older patients and subjected them to sessions of four cycles of 5 minutes of hypoxia followed by either normal oxygen or hyperoxia.[ref]
Intermittent hypoxia is also used for recovery after a spinal injury.[ref] It lowers cholesterol levels, decreases depression (animal study), and increases neurogenesis.[ref][ref][ref]
There is a saying for athletes, ‘live high, train low’, which uses the idea that changes in altitude and oxygen levels will affect performance. This is based on changes in HIF-1a, which causes an increase in EPO and red blood cells. Research does show that changing the oxygen content of the air – either through intermittent hypoxia or continuous hypoxia – can increase red blood cell production.[ref]
Timing and dose are vital with intermittent hypoxia therapy. Chronic intermittent hypoxia at night, or ‘sleep apnea’, has many negative consequences. In fact, sleep apnea is a risk factor for diabetes, obesity, and cardiovascular disease.[ref][ref] So, while a little intermittent hypoxia can be a good thing, chronic hypoxia every single night is bad.
So how can you do intermittent hypoxia therapy? There are athletic training facilities with expensive equipment to do it. And there are YouTube videos that have some odd DIY ideas. I’ll let you discover it on your own.
Exercise:
Getting your heart rate up and doing some aerobic exercise will elevate HIF-1a.[ref][ref]
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
NAD+ Reversing Aging? Overview of NR and NMN
Nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) are two supplements that have taken the longevity and anti-aging world by storm. With animal studies showing exciting results, including the reversal of age-related diseases, these supplements are an exciting glimpse into the future of reversing aging.
Is intermittent fasting right for you?
There are many internet docs and nutritional gurus promoting fasting as a way to lose weight and get healthy. The recommendations are often for intermittent fasting, for example, a 24-hour fast every week, or sometimes for longer fasts, like a week-long water fast. There are some real, science-based benefits to fasting.