Key takeaways:
~HMGB1 is a protein that has a unique role in activating the inflammatory response pathways.
~ Increased HMGB1 levels will call up the troops for a big response to an infection. HMGB1 plays a key role in the severe SARS-CoV-2 response by some people.
~ Genetic variants can increase or decrease the HMGB1 response.
HMGB1: From nuclear protein to inflammatory alarmin
HMGB1 (high mobility group box 1) is a multi-functional protein that has several roles, depending on where it is located in the body[ref]:
HMGB1 in the cell nucleus: Inside the nucleus, HMGB1 helps to organize the DNA, rearranging it so that different genes can be translated into their proteins. It is an important chromatin protein found in all cells. (I’ll explain further in just a minute…)
HMGB1 in the cytosol: Cellular cytosol is within the cell but outside of the nucleus. Under certain conditions, such as when there is oxidative stress or a bacterial pathogen present, HMGB1 moves from the nucleus into the cytosol.
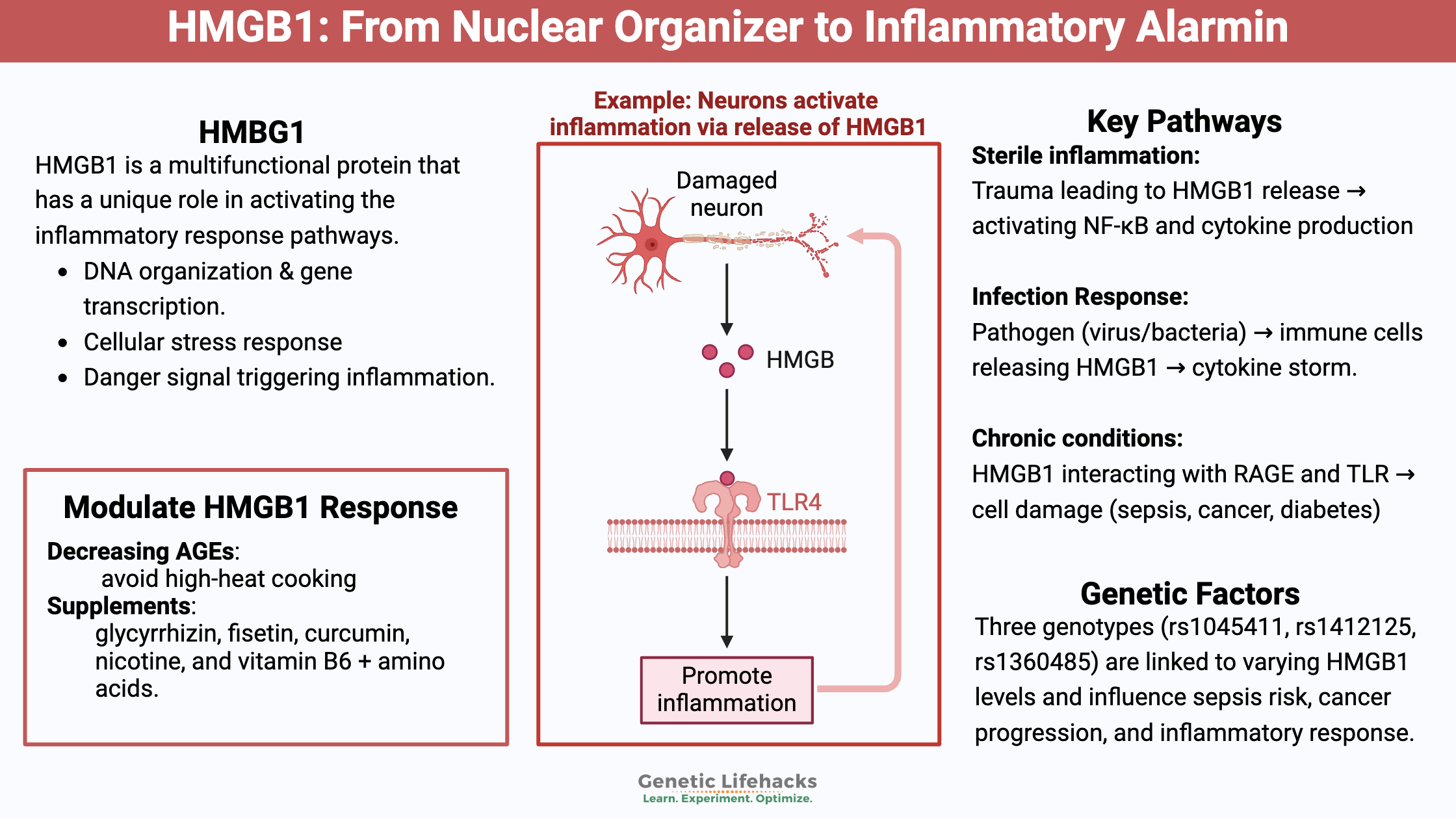
HMGB1 in the serum: The HMGB1 protein is released outside of the cell during trauma, alerting the body to the damage, thus causing a cascade of inflammatory events. Additionally, when inflammatory cytokines are abundant due to a viral or bacterial pathogen, HMGB1 also will be released from immune system cells via extracellular vesicles.[ref] This is referred to as HMGB1 acting as an alarmin or danger signal.
More about HMGB1 in the nucleus:
DNA must be packaged up tightly so it fits inside the nucleus of cells. But, for any cellular protein to be transcribed and translated, specific segments of the DNA have to open up and be available. There are several ways to package, unpack, and mark genes for translation.
The HMGB1 protein is one way this unpacking of DNA can occur — via loosening the packed DNA and bending it so that a gene can be translated. It is part of a family of HMG proteins involved in the way that DNA is unpacked to allow for translation.[ref]
All is good when HMGB1 is in the nucleus, doing its job of translating genes.
HMGB1 gets interesting, though, when it comes to all of its other roles in the body…
HMGB1 and Sterile Inflammation:
HMGB1’s activation occurs in response to damage to a tissue, such as after trauma. It is a molecule for sterile inflammation. When someone gets hurt, the levels of HMGB1 in the plasma rise within 6 hours after the trauma.[ref][ref]
Like everything in the immune response, this needs to be kept in balance.
High levels of HMGB1 are associated with multisystem organ failure after trauma.[ref]
After the release of HMGB1, it binds with the receptor for advanced glycation end products (RAGE), activating cytokines such as NF-κB. This starts a feedback loop, increasing the production of several different inflammatory cytokines.[ref]
We’ll circle back to RAGE and advanced glycation end products in just a minute…
HMGB1 and Inflammation due to Infections:
HMGB1 is also important for infections, acting as a pro-inflammatory alarmin or danger signal when the body has been infected by a pathogen.
- Lipopolysaccharide (LPS), which is present on the outside of certain types of bacterial cells, triggers the release of HMGB1 from immune system cells.[ref]
- Interferons, an immune system molecule released due to a viral infection, can also activate the release of HMGB1.
Macrophages, monocytes, and dendritic cells secrete HMGB1 as a mediator of inflammation. It is a signal – an alarm – that something is wrong and sets off an increased immune response.
Inflammation always needs to be kept in balance – enough response to fight off pathogens but tempered to limit the damage. You don’t want to end up with sepsis or ARDS.
Researchers have found, though, that while HMGB1 acts as a pro-inflammatory signal initially, later in an infection, the large quantities can act as an immunosuppressant. This can lead to a dysregulation of the inflammatory response.[ref]
Delayed:
In sepsis, HMGB1 levels rise (up to 300-fold) and reach a plateau more than a day after the onset of septic shock.[ref]
Localized:
In peritonitis, researchers found the HMGB1 levels in the abdominal fluid were 10-fold higher than in plasma, indicating that the rise in HMGB1 is localized in the area of infection.[ref]
HMGB1 is important in mortality in viral-induced pneumonia also, such as from influenza A. Animal studies show that blocking HMGB1 reduces mortality considerably.[ref]
HMGB1 and Cancer:
Another side of the HMGB1 picture is its role in cancer.
Preventing cell death:
When cellular stress causes increased reactive oxygen species (ROS), HMGB1 can move out of the cell nucleus and into the cell. There it helps to sustain autophagy (the recycling of damaged cellular components) and prevents apoptosis (cell death).
That is all good — unless that cell has a cancerous mutation. In the case of cancerous mutations, you want the cell to undergo apoptosis and die.
In many cancer types, including breast, lung, and colon cancers, there is an increase in HMGB1.[ref]
Additionally, higher levels of HMGB1 protein can trigger a couple of genes involved in tumor metastasis.[ref]
Researchers are looking at different ways of targeting HMGB1 in cancerous cells.
Again, it is all about balance. There is a trade-off between protecting cells vs. preventing cancer cell proliferation.
HMBG1 and Histamine release from Mast Cells:
Histamine is known for its role in allergy symptoms, but this biogenic amine does more than just make your nose run from pollen.
Mast cells are a type of immune system cell that can react immediately to foreign proteins (allergens) and pathogens (viruses, bacteria). When mast cells are activated, they can release large amounts of histamine.
A recent study showed that histamine induces HMGB1 translocation from endothelial cells by binding to the H1 receptor. This explains how histamine release can cause a cascade of inflammation in the vascular system. Importantly, histamine acted to induce HMGB1 only through binding to H1 receptors.[ref] Many allergy medicines block H1 receptors.
HMGB1, AGEs, and RAGEs:
Advanced glycation end products (AGEs) and the receptor for advanced glycation end products (RAGEs) also interact with HMGB1.
This is a huge topic — and you can read more about AGEs and RAGEs here.
In a nutshell, glycation is the binding of a sugar molecule to a protein. This process is ongoing in the body, but excessive glycation causes problems. Additionally, glycation end products that accumulate in the body generate some of the damage associated with aging. AGEs and RAGEs are also increased in type 2 diabetes.[ref]
The HMGB1 protein binds to RAGE (receptor for AGE) in addition to the toll-like receptors (immune system receptors activated by LPS). Researchers show this by injecting HMGB1 into mice that are lacking various receptors (e.g., RAGE, TLR2) to see the response.[ref]
One way that HMGB1 interacts with RAGE is by binding to it in order to transport LPS into the lysosomes of a cell. The HMGB1 then causes the lysosome to release the contents into the cytosol, which activates a strong immune response inside the cell resulting in cell death. Like a little cell bomb being triggered. Again, this can be good in certain situations. But… over-activation, such as in sepsis, is bad.[ref]
Why is this interesting?
AGEs and RAGEs elevation occurs in people with diabetes.[ref] This combination may play a role in why people with diabetes are at an increased risk of severe COVID-19 as well as certain other viral and bacterial infections.
SARS-CoV-2 interaction with HMGB1:
Researchers performing a genome-wide CRISPR screen found HMGB1 as a critical component for SARS-CoV-2 replication.[ref]
Other research also shows that HMGB1 may be playing a key role in severe COVID-19 when the immune system is out of control.[ref]
Interferon, which the body produces to combat a virus, can increase HMGB1 release. Cellular stress or cell death, such as in lung cells infected by the virus, also causes increased HMGB1 release. The RAGE receptors needed for HMGB1 binding are found in abundance on the alveolar cells in the lungs.[ref]
Previous studies on severe respiratory infections, such as the flu or RSV, show high levels of HMGB1 release. Blocking HMGB1 can stop the severe response (at least in mice, cell studies, etc.).[ref][ref]
Again, this may be a matter of timing as to whether HMGB1 needs to be blocked or not. As referenced above, HMGB1 is important in the inflammatory response, but going overboard can cause problems.
HMGB1 and Autoimmune diseases such as lupus:
One more trick up HMGB1’s sleeve is that it also interacts with certain autoimmune conditions, possibly causing flare-ups in lupus.[ref]
- Some people produce anti-HMGB1 antibodies. In a study of lupus patients, about 23% had anti-HMGB1 antibodies, compared with only 5% of the people in the healthy control group.[ref]
- Another study shows HMGB1 elevation in people with lupus, compared with healthy controls.[ref]
- Mechanistically, lupus is a disease of systemic inflammation, and people with lupus don’t clear out apoptotic cellular debris very well. This causes an increased release of HMGB1.[ref]
Related article: Lupus – Genetics and Root Causes
HMGB1 and periodontal disease:
A final example of HMGB1 in inflammation: In gingivitis or periodontal disease, the bacteria and other inflammatory cytokines trigger the release of HMGB1.[ref][ref]
Initially, in periodontal disease, an increase occurs in epithelial cells that release cytokines. Animal studies show that HMGB1 release is a critical component of the inflammation that occurs in periodontal disease. Inhibiting HMGB1 with anti-HMGB1 stops inflammation and bone loss in periodontitis (animal study).[ref]
Again, this isn’t a case where all HMGB1 is bad. For example, the release of HMGB1 after tooth extraction helps to regulate the wound-healing process.[ref][ref] Instead, it is the chronic elevation in periodontal disease that seems to be the issue.
Related article: Genetics and periodontal disease
HMGB1 Genotype Report
Lifehacks:
Decreasing AGE and RAGE: High-heat cooking forms AGES
Cooking foods at high heat forms a Maillard reaction – it is that browning reaction that causes things to taste good, like grilled steaks or crispy cookies. Unfortunately, the Maillard reaction also causes an increase in the advanced glycation end products in food. This corresponds to an increase in AGE in people who eat foods cooked at high heat.
Related article: AGEs, RAGEs, and Lifehacks for preventing formation here.
5 Supplements that decrease HMGB1:
Related Articles and Topics: