Intermittent fasting (IF) seems to have taken the health and wellness industry by storm. However you define it, IF essentially means you don’t eat for a period of time (18 hours, a full day), and then you eat.
What is interesting about IF is that it can change the gene expression in different tissues in the body. Something as simple as ‘not eating’ can cause an upregulation of proteins associated with longevity.
This article digs into the recent research on intermittent fasting, focusing on how it changes gene expression.
What does science say about intermittent fasting?
It is easy to get caught up in the hype from the experiences people share regarding their new favorite diet or lifestyle hack. But the proof is in the pudding – or rather not eating the pudding? (I have no idea where that idiom comes from!)
The latest research on intermittent fasting shows several interesting things:
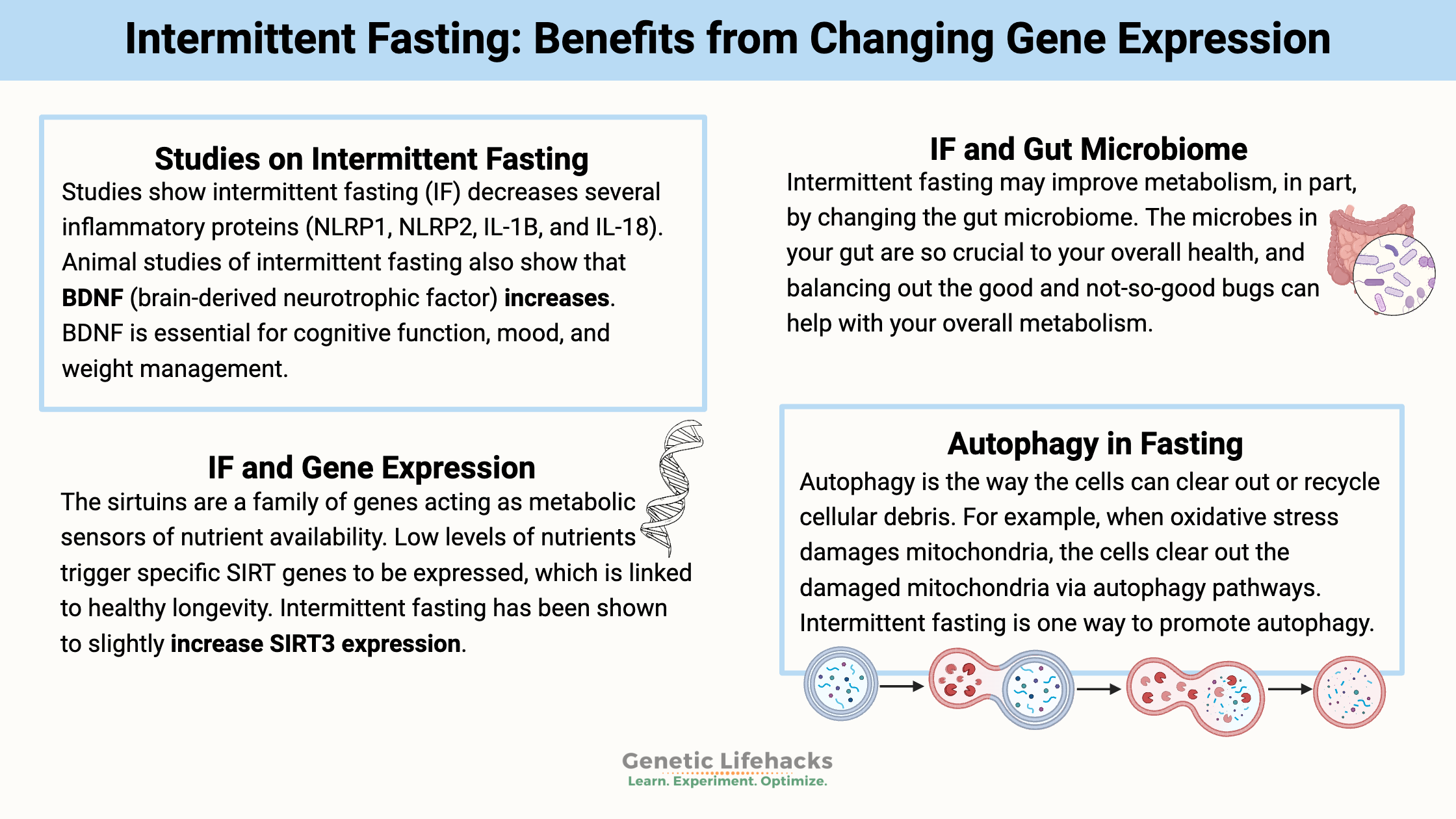
Intermittent fasting may improve metabolism, in part, by changing the gut microbiome.[ref] The microbes in your gut are so crucial to your overall health, and balancing out the good and not-so-good bugs can help with your overall metabolism.
IF also has decreased plasma insulin levels in a clinical trial.[ref] With so much of the population falling into the pre-diabetes category, this could be a great way for many to prevent diabetes.
Defining intermittent fasting:
There are many ways that researchers define intermittent fasting – from a 16-hour break in eating to a two-day-long fast. Additionally, there are ways to mimic the effects of fasting through restricting protein intake as well as benefits from time-restricted eating.
Here is a graphical overview (Creative Commons license) from a recent Frontiers in Genetics article.
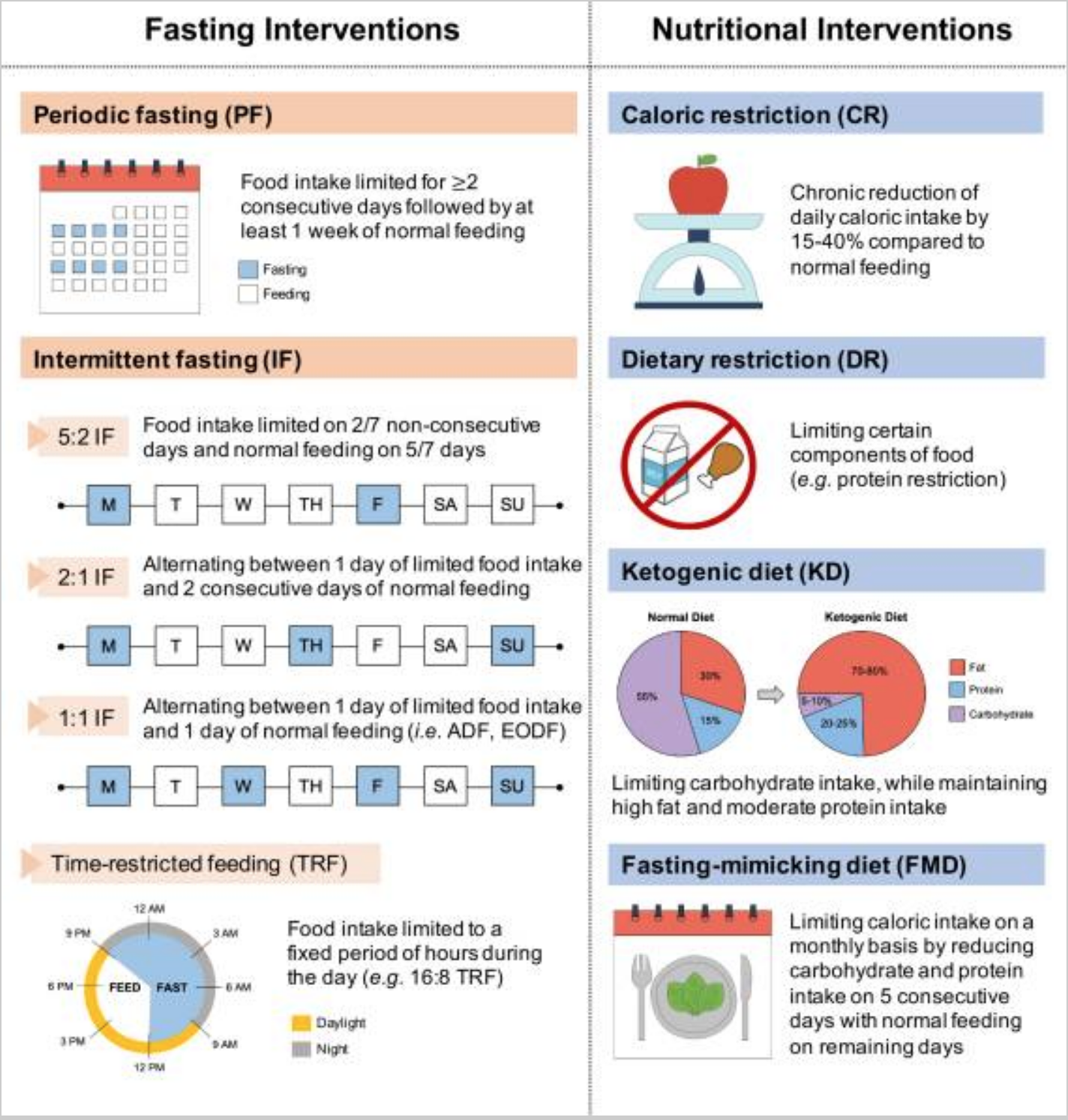
All of these methods of restricting food intake can affect gene expression and metabolic health.
Intermittent fasting and gene expression:
Digging into the ‘why’ and ‘how’ for intermittent fasting shows it changes the expression of many different genes. Essentially, your cells respond to the lack of nutrients by turning on and off different genes.
Why should you care about the changes in gene expression? Weight loss is one of the obvious effects of intermittent fasting. However, you may consider how the intervention affects specific biological pathways for your objectives.
Inflammation: A trial of intermittent fasting for four months in a mouse stroke model showed the expression of several inflammatory proteins (NLRP1, NLRP2, IL-1B, and IL-18) were decreased.[ref]
Longevity and health: The sirtuins are a family of genes acting as metabolic sensors of nutrient availability. Low levels of nutrients trigger specific SIRT genes to be expressed, which is linked to healthy longevity.[ref] (Read more about your SIRT gene variants)
Animal studies also show that decreasing calories and/or protein restriction will increase AMPK and decrease mTORC1, two proteins important for overall energy-sensing.[ref] Increasing AMPK is important for burning fat and making new mitochondria.[ref]
Brain health: Animal studies of intermittent fasting also show that BDNF (brain-derived neurotrophic factor) also increases.[ref] BDNF is essential for cognitive function, mood, and weight management. (Read more about your BDNF gene variants)
So what do human research studies show us about gene expression?
- A three-week-long intermittent fasting clinical trial showed a slight increase (~3%) in SIRT3.[ref]
- A fasting and refeeding trial of healthy adults showed that fasting decreased the NLRP3 inflammasome activation. Additionally, inflammatory gene expression for NF-κB, TNF, and IL1B was lower during fasting and higher after refeeding.[ref]
- A clinical trial comparing fasting in obese vs. normal-weight participants found several differences between the two groups. For example, AMPK activity was reduced in lean individuals, but no change in obese people. Additionally, the shift to burning fat for fuel was blunted in people who were obese.[ref]
- A clinical trial of early time-restricted eating (eating only between 8 am and 2 pm) showed SIRT1 gene expression was upregulated, as was the autophagy gene LC3A. Additionally, it increased BDNF expression in the evening.[ref]
Not all clinical trials on intermittent fasting show amazing results. A recent 8-week-long trial in healthy adults showed no significant differences between IF and a control group other than a little bit of weight loss in the IF group. The researchers looked at a number of different parameters, including BDNF, liver enzymes, blood pressure, mood, etc.[ref]
Another clinical trial in obese women found the markers of inflammation (TNFα, IL6, and IL10) were not changed from intermittent fasting or daily calorie restriction. In fact, inflammatory response increased in adipose tissue, possibly due to lipolysis.[ref]
(Check to see if you are likely to have genetically higher TNF-alpha)
What is autophagy in fasting?
In conjunction with intermittent fasting, a common term that you will see is ‘autophagy’.
Autophagy is the way the cells can clear out or recycle cellular debris. For example, when oxidative stress damages mitochondria, the cells clear out the damaged mitochondria via autophagy pathways, clearing the way to create new mitochondria.
Intermittent fasting is one way to promote autophagy.
People with diabetes often damage the insulin-producing beta cells in the pancreas. Animal studies show that intermittent fasting restores autophagic-flux to the beta cells in the pancreas. Clearing out damaged mitochondria and enhancing beta-cell survival is a good thing.[ref]
(Read more about your autophagy gene variants)
Should you do intermittent fasting if you are diabetic?
A recent randomized controlled trial on intermittent fasting in people with type 2 diabetes raises a couple of concerns. While the trial participants did have improvements in their weight, HbA1c, and fasting glucose, there was an increase in the rate of hypoglycemia.[ref]
If you have type 2 diabetes, talk with your doctor, and understand the risks of hypoglycemia when intermittent fasting. If your doctor isn’t familiar with the benefits (and risks) of intermittent fasting for type 2 diabetes, you could check out physician groups that specialize in IF for diabetes, such as Virta Health.
(Check out the free genetic risk report for diabetes)
Conclusion:
There are definite benefits for weight loss and metabolic health for most people who do intermittent fasting. But it may not be right for everyone, especially if you have problems with low blood sugar. Additionally, the impact on inflammatory markers may not be the same for everyone.
If you have medical questions about whether IF is right for you, talk with your doctor.
Related Articles and Topics:
Rapamycin, mTOR, and Your Genes
Rapamycin is an antibiotic used as an immunosuppressant, an anti-cancer agent, and to prevent blocked arteries. It is now the focus of longevity and healthspan-extending research through its inhibition of mTOR.
Telomere Length: How Your Genes Affect Telomeres and Aging
Your telomeres are the region at the end of each chromosome that keeps your DNA intact when your cells divide. Telomeres that are too short cause cells to stop dividing. It causes some diseases of aging. Genetics plays a role here – along with diet and lifestyle. (Member’s only article)
Preventing Alzheimer’s Disease
Billions of dollars have been spent in the last couple of decades on trying to find drugs to stop the tangled accumulation of beta-amyloid plaque without much success. A new direction of research is looking into the ties between circadian rhythm dysfunction and Alzheimer’s disease.
Blood glucose levels: how your genes impact blood sugar regulation
Genetics plays a significant role in your blood glucose regulation. Discover your genetic susceptibility to blood sugar problems to help with blood glucose stability.
References:
An, Ping, et al. “Role of APOBEC3F Gene Variation in HIV-1 Disease Progression and Pneumocystis Pneumonia.” PLoS Genetics, vol. 12, no. 3, Mar. 2016, p. e1005921. PubMed, https://doi.org/10.1371/journal.pgen.1005921.
Asif, Shaza, et al. “Understanding Dietary Intervention-Mediated Epigenetic Modifications in Metabolic Diseases.” Frontiers in Genetics, vol. 11, Oct. 2020, p. 590369. PubMed Central, https://doi.org/10.3389/fgene.2020.590369.
Baik, Sang-Ha, et al. “Intermittent Fasting Increases Adult Hippocampal Neurogenesis.” Brain and Behavior, vol. 10, no. 1, Jan. 2020, p. e01444. PubMed, https://doi.org/10.1002/brb3.1444.
Bogdanova, Natalia, et al. “Hereditary Breast Cancer: Ever More Pieces to the Polygenic Puzzle.” Hereditary Cancer in Clinical Practice, vol. 11, no. 1, Sept. 2013, p. 12. PubMed Central, https://doi.org/10.1186/1897-4287-11-12.
Chen, Zhishan, et al. “Integrative Genomic Analyses of APOBEC-Mutational Signature, Expression and Germline Deletion of APOBEC3 Genes, and Immunogenicity in Multiple Cancer Types.” BMC Medical Genomics, vol. 12, Sept. 2019, p. 131. PubMed Central, https://doi.org/10.1186/s12920-019-0579-3.
Compaore, Tegwinde Rebeca, et al. “APOBEC3G Variants and Protection against HIV-1 Infection in Burkina Faso.” PloS One, vol. 11, no. 1, 2016, p. e0146386. PubMed, https://doi.org/10.1371/journal.pone.0146386.
Corley, B. T., et al. “Intermittent Fasting in Type 2 Diabetes Mellitus and the Risk of Hypoglycaemia: A Randomized Controlled Trial.” Diabetic Medicine: A Journal of the British Diabetic Association, vol. 35, no. 5, May 2018, pp. 588–94. PubMed, https://doi.org/10.1111/dme.13595.
Deng, Ya, et al. “Intermittent Fasting Improves Lipid Metabolism Through Changes in Gut Microbiota in Diet-Induced Obese Mice.” Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, vol. 26, Nov. 2020, p. e926789. PubMed, https://doi.org/10.12659/MSM.926789.
Fann, David Yang-Wei, et al. “Intermittent Fasting Attenuates Inflammasome Activity in Ischemic Stroke.” Experimental Neurology, vol. 257, July 2014, pp. 114–19. PubMed, https://doi.org/10.1016/j.expneurol.2014.04.017.
Hanjani, Nazanin Asghari, and Mohammadreza Vafa. “Protein Restriction, Epigenetic Diet, Intermittent Fasting as New Approaches for Preventing Age-Associated Diseases.” International Journal of Preventive Medicine, vol. 9, June 2018, p. 58. PubMed Central, https://doi.org/10.4103/ijpvm.IJPVM_397_16.
He, Xiu-Ting, et al. “Association between Polymorphisms of the APOBEC3G Gene and Chronic Hepatitis B Viral Infection and Hepatitis B Virus-Related Hepatocellular Carcinoma.” World Journal of Gastroenterology, vol. 23, no. 2, Jan. 2017, pp. 232–41. PubMed, https://doi.org/10.3748/wjg.v23.i2.232.
Iqbal, Khurshid, et al. “Correlation of Apolipoprotein B MRNA-Editing Enzyme, Catalytic Polypeptide-like 3G Genetic Variant Rs8177832 with HIV-1 Predisposition in Pakistani Population.” Current HIV Research, vol. 16, no. 4, July 2018, pp. 297–301. PubMed Central, https://doi.org/10.2174/1570162X16666181018155827.
Jamshed, Humaira, et al. “Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans.” Nutrients, vol. 11, no. 6, May 2019, p. E1234. PubMed, https://doi.org/10.3390/nu11061234.
Kessler, Christian S., et al. “A Nonrandomized Controlled Clinical Pilot Trial on 8 Wk of Intermittent Fasting (24 h/Wk).” Nutrition (Burbank, Los Angeles County, Calif.), vol. 46, Feb. 2018, pp. 143-152.e2. PubMed, https://doi.org/10.1016/j.nut.2017.08.004.
Liu, Bo, et al. “Markers of Adipose Tissue Inflammation Are Transiently Elevated during Intermittent Fasting in Women Who Are Overweight or Obese.” Obesity Research & Clinical Practice, vol. 13, no. 4, Aug. 2019, pp. 408–15. PubMed, https://doi.org/10.1016/j.orcp.2019.07.001.
Liu, Haiyan, et al. “Intermittent Fasting Preserves Beta-Cell Mass in Obesity-Induced Diabetes via the Autophagy-Lysosome Pathway.” Autophagy, vol. 13, no. 11, Nov. 2017, pp. 1952–68. PubMed Central, https://doi.org/10.1080/15548627.2017.1368596.
Luo, Yiqiao, et al. “Sulforaphane Inhibits the Expression of Long Noncoding RNA H19 and Its Target APOBEC3G and Thereby Pancreatic Cancer Progression.” Cancers, vol. 13, no. 4, Feb. 2021, p. 827. PubMed Central, https://doi.org/10.3390/cancers13040827.
Mourier, Tobias, et al. “Host-Directed Editing of the SARS-CoV-2 Genome.” Biochemical and Biophysical Research Communications, vol. 538, Jan. 2021, pp. 35–39. PubMed Central, https://doi.org/10.1016/j.bbrc.2020.10.092.
Park, Charny, et al. “Integrative Molecular Profiling Identifies a Novel Cluster of Estrogen Receptor-Positive Breast Cancer in Very Young Women.” Cancer Science, vol. 110, no. 5, May 2019, pp. 1760–70. PubMed, https://doi.org/10.1111/cas.13982.
Poulain, Florian, et al. “Footprint of the Host Restriction Factors APOBEC3 on the Genome of Human Viruses.” PLOS Pathogens, vol. 16, no. 8, Aug. 2020, p. e1008718. PLoS Journals, https://doi.org/10.1371/journal.ppat.1008718.
Robertson, Lauren T., et al. “Protein and Calorie Restriction Contribute Additively to Protection from Renal Ischemia Reperfusion Injury Partly via Leptin Reduction in Male Mice.” The Journal of Nutrition, vol. 145, no. 8, Aug. 2015, pp. 1717–27. PubMed, https://doi.org/10.3945/jn.114.199380.
Sadeghpour, Shiva, et al. “Human APOBEC3 Variations and Viral Infection.” Viruses, vol. 13, no. 7, July 2021, p. 1366. PubMed Central, https://doi.org/10.3390/v13071366.
Sadler, Holly A., et al. “APOBEC3G Contributes to HIV-1 Variation through Sublethal Mutagenesis.” Journal of Virology, vol. 84, no. 14, July 2010, pp. 7396–404. PubMed Central, https://doi.org/10.1128/JVI.00056-10.
Sharma, Shraddha, et al. “APOBEC3A Cytidine Deaminase Induces RNA Editing in Monocytes and Macrophages.” Nature Communications, vol. 6, Apr. 2015, p. 6881. PubMed Central, https://doi.org/10.1038/ncomms7881.
Stavrou, Spyridon, and Susan R. Ross. “APOBEC3 Proteins in Viral Immunity.” Journal of Immunology (Baltimore, Md. : 1950), vol. 195, no. 10, Nov. 2015, pp. 4565–70. PubMed Central, https://doi.org/10.4049/jimmunol.1501504.
Sui, Shuang, et al. “Correlation of APOBEC3G Polymorphism with Human Papillomavirus (HPV) Persistent Infection and Progression of Cervical Lesions.” Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, vol. 25, Sept. 2019, pp. 6990–97. PubMed Central, https://doi.org/10.12659/MSM.916142.
Traba, Javier, et al. “Fasting and Refeeding Differentially Regulate NLRP3 Inflammasome Activation in Human Subjects.” The Journal of Clinical Investigation, vol. 125, no. 12, pp. 4592–600. PubMed Central, https://doi.org/10.1172/JCI83260. Accessed 3 June 2022.
Wegman, Martin P., et al. “Practicality of Intermittent Fasting in Humans and Its Effect on Oxidative Stress and Genes Related to Aging and Metabolism.” Rejuvenation Research, vol. 18, no. 2, Apr. 2015, pp. 162–72. PubMed Central, https://doi.org/10.1089/rej.2014.1624.
Wijngaarden, Marjolein A., et al. “Effects of Prolonged Fasting on AMPK Signaling, Gene Expression, and Mitochondrial Respiratory Chain Content in Skeletal Muscle from Lean and Obese Individuals.” American Journal of Physiology-Endocrinology and Metabolism, vol. 304, no. 9, May 2013, pp. E1012–21. journals.physiology.org (Atypon), https://doi.org/10.1152/ajpendo.00008.2013.
Zhu, Yueming, et al. “Metabolic Regulation of Sirtuins upon Fasting and the Implication for Cancer.” Current Opinion in Oncology, vol. 25, no. 6, Nov. 2013, pp. 630–36. PubMed Central, https://doi.org/10.1097/01.cco.0000432527.49984.a3.