Key Takeaways:
~ The formation of steroid hormones, such as testosterone and estrogen, depends on CYP17A1.
~ Genetic variants (SNPs) in the CYP17A1 gene can impact hormone levels.
~ Knowing your genetic variants can help you understand your risk for hormone-related conditions.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
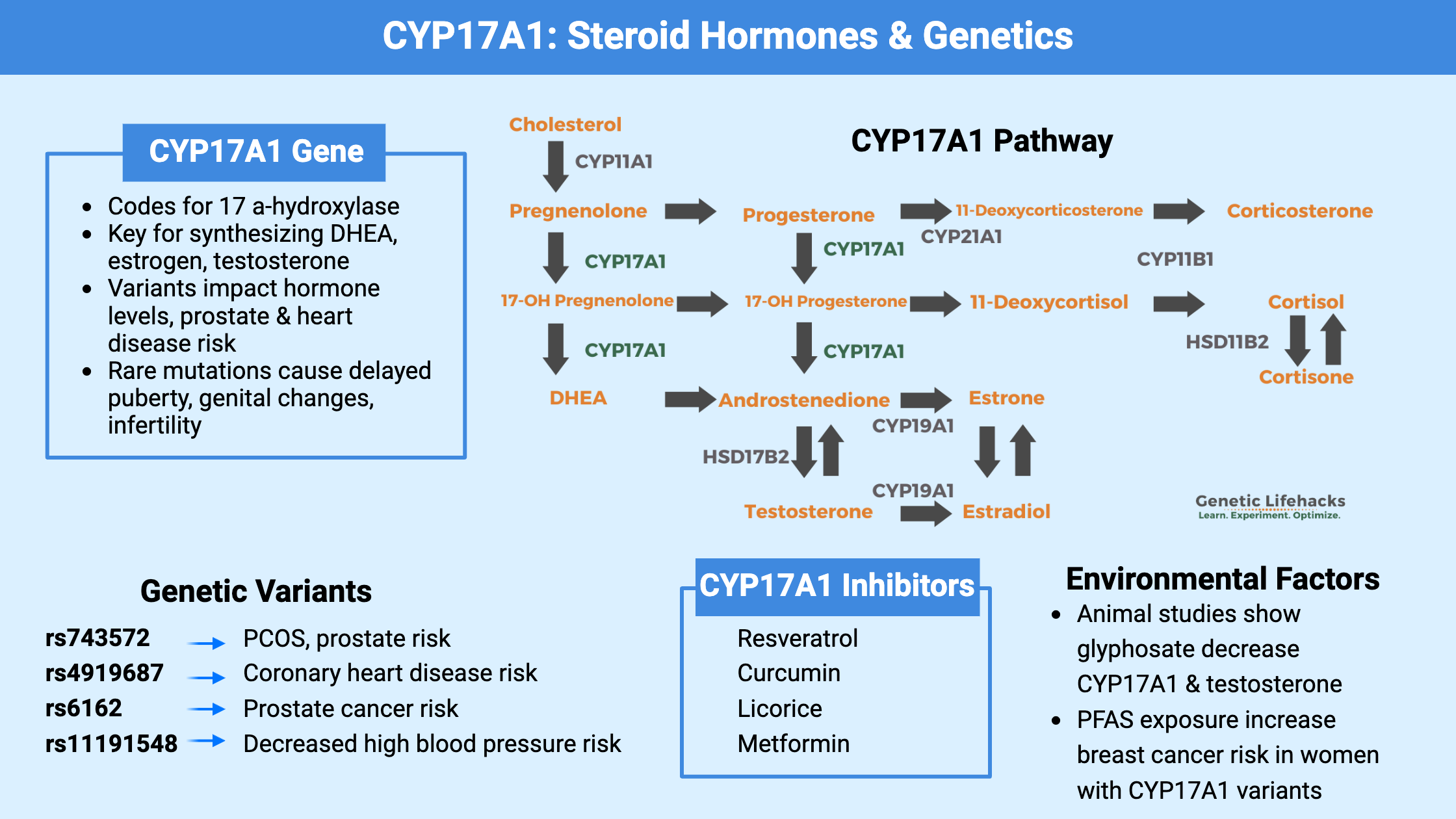
What is CYP17A1?
The CYP17A1 gene encodes an enzyme called 17 α-hydroxylase. It is an essential enzyme needed for the formation of steroid hormones, including the precursors for testosterone and estrogen production.
Cholesterol is the precursor for all of the sex hormones and steroid hormones (testosterone, estradiol (estrogen), progesterone, cortisol, and aldosterone). Cholesterol is first converted into pregnenolone, which can then be converted to 17OH-pregnenolone or progesterone by the CYP17A1 enzyme. These key molecules can then be converted as needed into other hormones.
Located primarily in the gonads and adrenal glands, CYP17A1 is responsible for catalyzing two key reactions in steroidogenesis: 17α-hydroxylation and 17,20-lyase activity.
Here is an overview of the pathways that are impacted by CYP17A1.
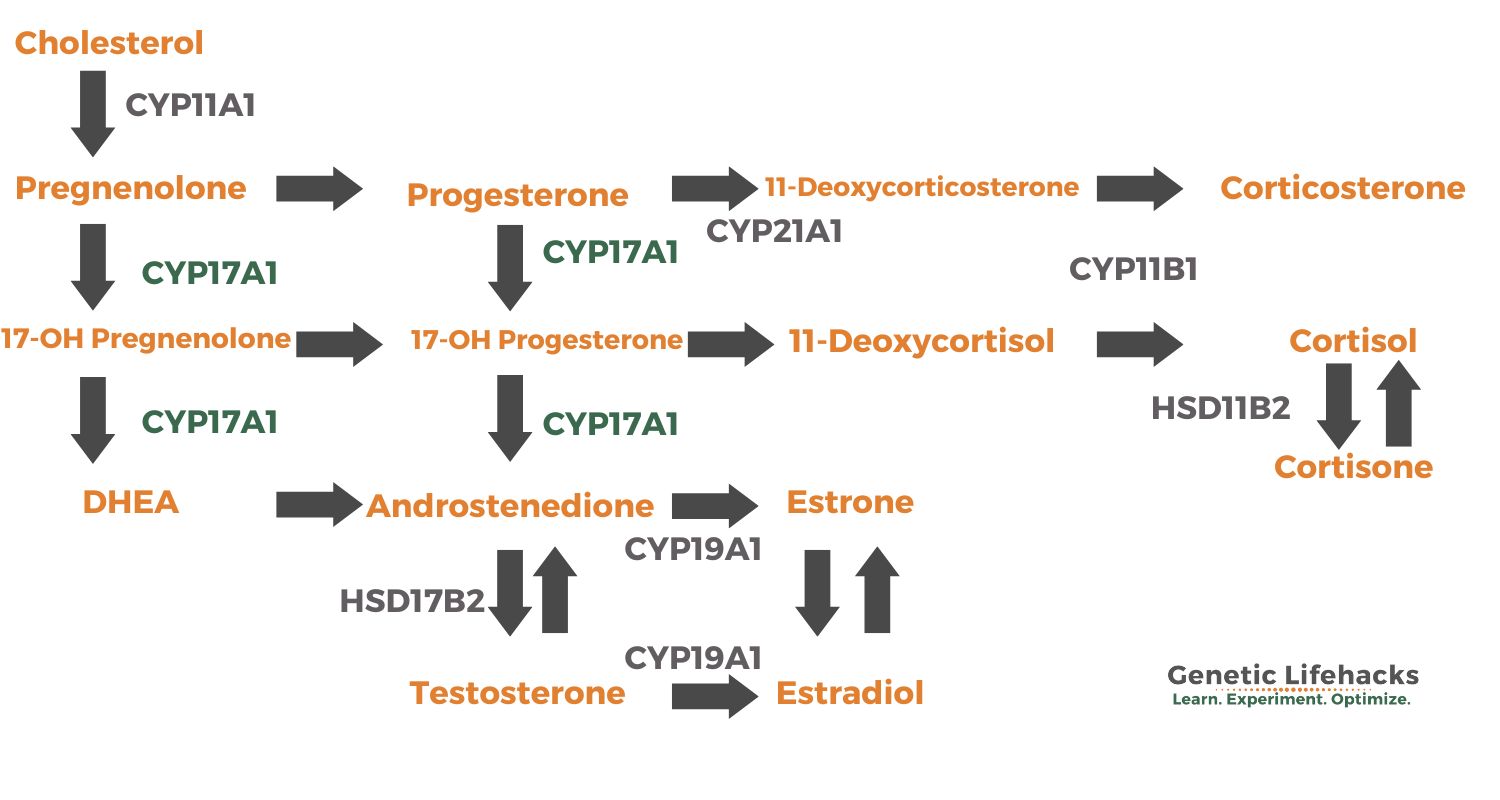
The 17α-hydroxylase activity of CYP17A1 is responsible for the conversion of pregnenolone and progesterone to their derivatives. These intermediates serve as precursors for the synthesis of cortisol and other glucocorticoids. This mainly takes place in the adrenal glands.
On the other hand, the 17,20-lyase activity of CYP17A1 is involved in the production of androgens, such as dehydroepiandrosterone (DHEA) and androstenedione. These androgens act as precursors for the synthesis of testosterone and estrogens.
The activity of CYP17A1 is tightly regulated and plays a critical role in several physiological processes, including sexual development, fertility, and the maintenance of hormonal balance. Feedback loops involving cortisol and the sex steroid hormones regulate the production of ACTH (Adrenocorticotropic Hormone ) and LH (Luteinizing Hormone), which then in turn control the levels of CYP17A1.
However, genetic variations in the CYP17A1 gene can influence its function, leading to altered hormone production. These variants can also interact with environmental toxins to further alter CYP17A1 levels, leading to downstream effects on sex hormones, fertility, and overall health.
Interactions with environmental factors:
Glyphosate (commonly used herbicide):
A 2021 study in animals showed that glyphosate decreases testosterone by decreasing the expression of CYP17A1 in Leydig cells, which are the primary site of testosterone production. The study stated: “Notably, serum testosterone levels were also drastically reduced in mice treated with glyphosate.” [ref] However, this may not hold true for all herbicides formulated with glyphosate. The various different ingredients in commercially available herbicides interact with glyphosate, and one herbicide formulation increased CYP17A1 in rats.[ref]
Steroid hormone levels are extremely important during the development of a fetus, and exposure to toxicants during development may have effects that aren’t fully understood until puberty.
Organochloride pesticides:
A mother’s CYP17A1 genotype may also be important, as estrogen levels impact the growth of the fetus. One study found an interaction between exposure to organochlorine pesticides, the normal CYP17A1 variant, and a lower birth weight.[ref] In this case, the variant allele increases CYP17A1 activity and the wildtype or typical CYP17A1 allele is associated with lower activity.
PFAS (perfluoroalkyl substances):
PFAS is a ubiquitous environmental pollutant found in non-stick coatings, fabric stain guards, personal care products, and food packaging. In mothers with higher PFAS levels, researchers found that their infants had altered CYP17A1 expression.[ref]
What do genetic changes in CYP17A1 cause?
Rare mutations in CYP17A1: 17α-hydroxylase deficiency
To determine the function of a gene, scientists often create animal models with nonfunctioning or ‘knock out’ genes. This helps researchers understand the effects of the gene.
In mice, a genetic deficiency of CYP17A1 causes all mice (including those with a Y chromosome) to appear female. They were also all infertile and had abnormal genitalia and reproductive organs.
In the CYP17A1 knockout mice with XY chromosomes, there was a complete lack of testosterone, and in the KO mice with XX chromosomes, there was an accumulation of progesterone.[ref]
Similarly, rare mutations in CYP17A1 in humans cause 17α-hydroxylase deficiency. This condition occurs in about 1 in 1,000,000 individuals who have two mutated CYP17A1 genes.
A case series describing individuals with 17α-hydroxylase deficiency shows that there is a range of phenotypes and severity due to CYP17A1 mutations. In general, genetically male (XY) individuals will have more female-appearing external genitalia. Both males and females with 17α-deficiency have delayed puberty, are infertile, and often have hypertension at a young age.[ref]
Common variants in CYP17A1:
Quite a few common polymorphisms (SNPs) are found in the CYP17A1 gene, and researchers have identified them as disease risk factors for heart disease, breast cancer, and prostate cancer. Please keep in mind that the variants alone don’t cause the disease, but rather they add to the susceptibility and risk for the disease.
Heart Disease:
While much of the research on CYP17A1 has focused on hormone-related cancers or developmental problems, estrogen and steroid hormones also affect the heart. In women, higher levels of estrogen are thought to protect against heart disease, while the opposite may be true for men.[ref] In the genotype report, you’ll see that several of the CYP17AA1 variants are linked to heart-related changes.
Prostate cancer:
CYP17A1 and the conversion of androgens can be an important factor in prostate cancer prognosis. A CYP17A1 inhibitor (abiraterone) was approved by the FDA a few years ago for the treatment of late-stage prostate cancer treatment [ref].
Breast Cancer, toxin exposure, and CYP17A1:
Research shows that exposure to PFAA (perfluoroalkyl acids) increases the relative risk of breast cancer. However, the increased risk may only apply to people with certain CYP17A1 genotypes. A recent study found that a CYP17A1 rs743572 variant (below in the genotype report), which is associated with higher enzyme function, was associated with a decreased risk of breast cancer at higher PFAA exposure levels.[ref]
Similarly, higher levels of BPA are associated with an increased relative risk of breast cancer. Among women with the CYP17A1 rs743572 variant, which leads to higher function, there was no increased risk of breast cancer from BPA. Women with the lower CYP17A1 variants had a 2.5-fold increased relative risk of breast cancer with higher BPA exposure.[ref]
CYP17A1 Genotype Report:
Lifehacks: Lifestyle changes and supplements that impact CYP17A1
In general, balanced expression of CYP17A1 is needed — an excess of hormones can lead to cancer growth, while not enough of the hormones can have long-term health effects. The research on genetic variants that interact with BPA, PFAS, or other environmental factors clearly shows that some people are going to be more affected than others.
4 Natural Inhibitors of CYP17A1:
Related Articles and Topics:
Estrogen: How it is made and how it is eliminated
Find out how genes influence the creation and the breakdown pathways for estrogens.
Testosterone Genes
Genetic variants play a role in testosterone production and circulating levels.
PCOS: Personalized Solutions
There is more to PCOS than just altered hormones. Learn how your genes are impacting your root cause.