Key takeaways:
~ Melanopsin is a receptor that is activated by specific wavelengths of light.
~ It is found in the retina of the eye and controls circadian rhythms and melatonin production.
~ Melanopsin is also found in the skin, blood vessels, and adipose tissue, and exposure to blue-wavelength light can also affect these tissues.
~ Genetic variants in the melanopsin (OPN4) gene affect mood, seasonal affective disorder, and sensitivity to blue light.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.
What is melanopsin?
Melanopsin is a type of opsin that belongs to a family of G protein-coupled receptors (GPCRs). The other opsins are best known for their role in rods and cones in the retina, which are involved in vision.
Opsins are receptors that change their signaling state when activated by light absorption at specific wavelengths. Incorporated within the opsin receptor is a vitamin A derivative that absorbs a photon from light. The capture of the photon causes the physiological response – the change within the cell resulting from the activation of the receptor.
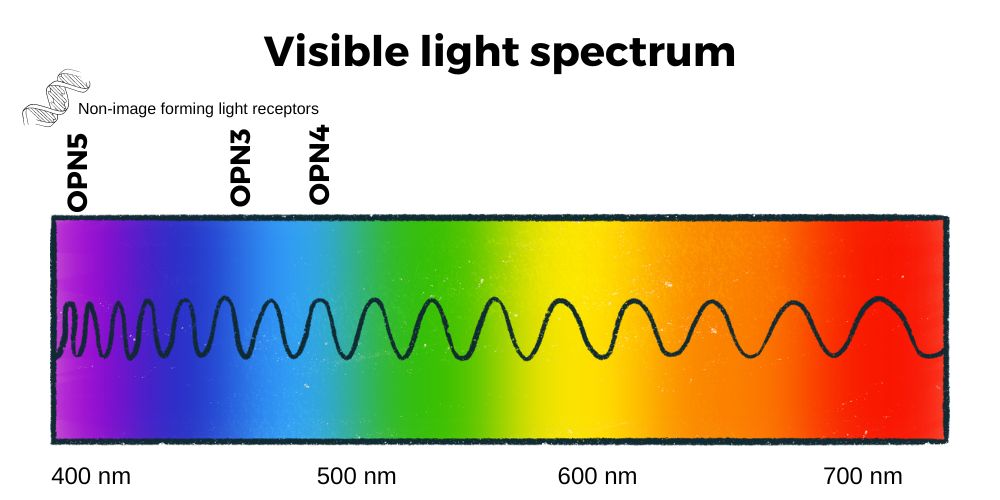
The wavelength of light in the visible spectrum ranges from about 400 nm (violet) to about 700 nm at the transition between red and infrared. Blue and violet light have higher photon energy than other wavelengths.[ref]
For the opsins in the rods and cones, the different wavelengths of light activate the rods and cones and send a signal that gets interpreted as a visual image in the brain. This is pretty cool… but what is even cooler is that we also have non-image-forming opsins, such as melanopsin.
Activation of melanopsin (OPN4 gene) in the retina by light in the blue wavelengths (~480 nm) sets our circadian clock each day. It is also involved in the pupillary light response and photophobia or light aversion.[ref]
Melanopsin is also involved in task-evoked pupillary responses, which are the changes in pupil size that correlate with concentration, decision-making, and increased cognitive load.[ref] In other words, when you are mentally focused, your pupil size changes and is controlled by melanopsin. In animal studies, researchers have found that exposure to daylight interacts with melanopsin to improve mood.[ref]
Where is melanopsin found?
Melanopsin is encoded by the OPN4 gene, and researchers have determined which tissues express the OPN4 gene to understand where melanopsin is active.
Currently, research indicates that melanopsin is active as a receptor in:
- the retina of the eye
- adipose (fat) tissue
- skin
- smooth muscle around blood vessels, lungs
- certain areas of the brain
Melanopsin in the eye:
Melanopsin is found primarily in a subset of retinal ganglion cells (RGCs) in the eye. Unlike the rods and cones which are responsible for vision, these melanopsin-containing cells are involved in non-image-forming photoreception.
As a non-image-forming photoreceptor, the activation of melanopsin by light in the blue wavelengths directly signals to the brain that it is daytime. This triggers a cascade of cellular changes based on circadian clock genes, which control about 40% of the processes that take place in cells. Many cells have different functions and responses depending on whether it is day or night.
Essentially, activation of melanopsin receptors in the eye sends a signal to the region of the hypothalamus called the suprachiasmatic nucleus. This tiny region in the brain controls the core circadian clock, which then influences circadian rhythm throughout the body. The changing wavelength (color) of light during the day and the absence of light at night sets the circadian clock in the suprachiasmatic nucleus.
Animal studies show that melanopsin is important for eye development in the embryo. In mice with the OPN4 gene deleted, the eyes develop with a myopic shift.[ref] (I’m envisioning mice that need to wear tiny glasses.)
A recent study also showed that melanopsin may play a role in visual perception by slightly enhancing peripheral vision during visual fixation.[ref]
Activating the melanopsin receptor in the eye turns off melatonin production in the pineal gland. Melatonin is produced in large quantities by the pineal gland at night, and activation of the melanopsin receptor by blue light is like an off switch for nocturnal melatonin production.
Studies in mice show that activation of the melanopsin receptors, even in mice without vision, alters both behavior and mood.[ref]
Keep in mind that until the advent of electric lights, no one was exposed to light in the blue spectrum except during the day. All plants and animals (and even bacteria) have developed circadian rhythms based on light during the day and a lack of light at night. Electric lights – especially LED and fluorescent lights – now expose humans (and insects, animals, plants, etc) to light in the blue wavelengths at night. Fire-based light sources in the past all provide yellow and red spectrums of light.
I’ll cover this more in the circadian section of the Lifehacks section — this is an area where you can have a big impact.
In white adipose tissue:
Researchers have discovered that melanopsin is found in your white adipose tissue — yes, your fat tissue has blue light receptors.
Up to 5% of light in the blue and green wavelengths can penetrate the skin layers and reach subcutaneous fat, but only a portion of the fat cells react to blue light (10-13% in one study).[ref] Other studies in animals show that light can penetrate past the subcutaneous fat tissue, perhaps affecting visceral fat and internal organs as well.[ref]
Researchers found that when the melanopsin receptor in fat cells is activated by daily exposure to blue light, a stable current is generated. This caused an increase in lipolysis, which is the use of fat for cellular energy. In the experiment, adipose tissue was exposed to blue light for 4 hours a day for 14 days. The increase in lipolysis and decrease in fat cell size was significant by day 11 compared to control cells in the dark. The fat cells also secreted lower levels of leptin and adiponectin.[ref]
Does this mean that we should all run around naked in the sunshine for four hours a day to lose weight? That is likely overly simplistic. For one, it doesn’t take into account other light wavelengths from sunlight, especially since UV wavelengths also impact fat cells in other ways.[ref] However, the study results could mean that researchers who have been doing cell studies on adipose tissue exposed to fluorescent lights may get inaccurate results sometimes.
In small blood vessels:
Melanopsin, or expression of the OPN4 gene, is also found in blood vessels. Researchers have known since the 1950s that exposure to blue wavelength light causes blood vessels near the skin’s surface to relax. Using mice with the OPN4 gene deleted, they discovered that melanopsin is the receptor responsible for how blue light can cause relaxation in blood vessels and changes in blood flow.[ref]
Interestingly, the relaxation of blood vessels with blue light activation of OPN4 does not involve nitric oxide. The release of nitric oxide in the skin from sunlight is through a separate pathway.
In the skin:
Melanopsin is also found in the melanocytes in the skin. Animal studies show that melanopsin in the skin helps to protect against damage from UVA radiation.[ref] Melanopsin may play a protective role against melanoma. In human melanoma cell studies, OPN4 expression is downregulated, compared to normal skin cells.[ref]
One study explains that “the light signal is converted to an intracellular signal via OPN4 in the human skin.” The melanopsin receptor is activated in an intensity-dependent manner.[ref]
In the brain:
Researchers have found OPN4 gene expression in brain samples from forensic autopsies. OPN4 was found mainly in central areas of the brain in membranous compartments and cytoplasmic vesicles. This finding raised questions, of course, as to why a light sensor is in the brain – and what is it doing.[ref] More research is needed here to understand if light penetrates the skull and reaches the brain.
Are there other non-image-forming opsins?
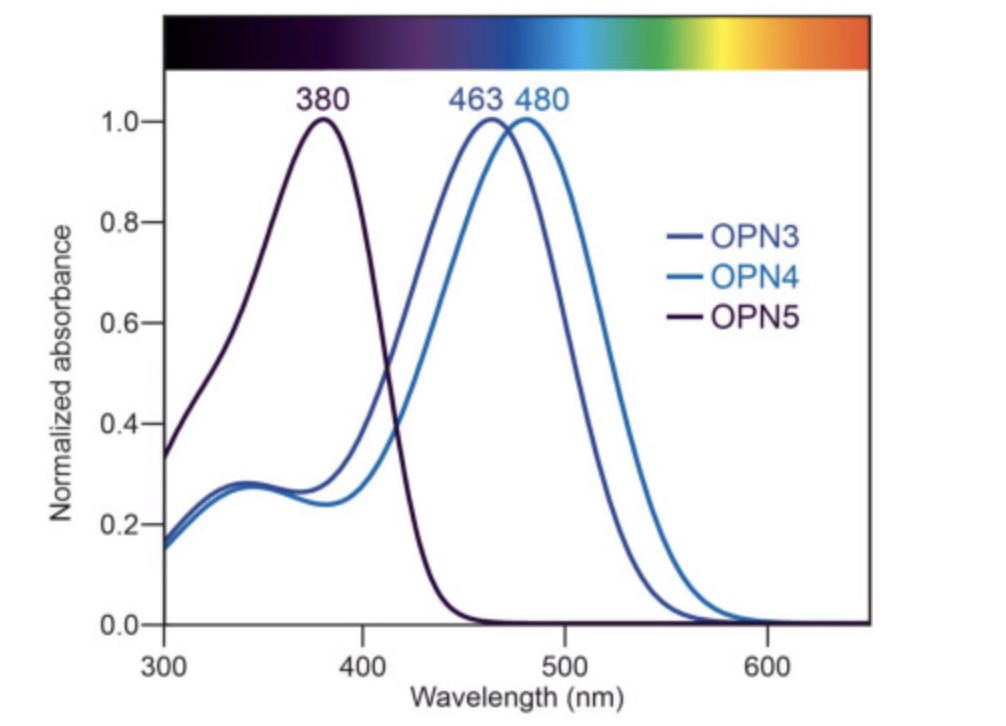
In addition to melanopsin (OPN4), there is also encephalopsin (OPN3 gene) and neuropsin (OPN5).
OPN3 is also found in adipose tissue and responds to light in the blue wavelengths (~465 nm). Mice lacking the OPN3 gene become more obese and insulin resistant than normal when fed a high-fat diet, but they don’t show changes on normal mouse chow. The lack of OPN3 also seems to decrease heat production due to stress and plays a role in thermogenesis.[ref]
Other animal studies show that OPN3 is also expressed in the brain, eyes, olfactory system, heart, lungs, skin, immune system, and several abdominal organs. Human studies show that OPN3 is expressed in immune cells, lungs, smooth muscle, skin, blood vessels, brain, and eyes.[ref]
OPN3 has recently been shown to be involved in the relaxation of the lung smooth muscle in the presence of blue light (~450 nm).[ref] Genetic variants in OPN3 have been associated with asthma susceptibility in multiple studies, tying into the relaxation of the airway muscles.[ref]
OPN5 encodes neuropsin, which is sensitive to visible violet light wavelengths (~380 nm). It is found in the retina, skin, brain, testes — and also in adipose tissue.
Studies in mice show that OPN5 in adipose tissue and the brain is important in thermoregulation during cold exposure.[ref] In birds, OPN5 controls the ovarian hormones involved in egg production. Changes in the length of daylight are detected by OPN5, which then controls reproductive hormones involved in seasonal egg production. Why are there so many studies on this? Well, researchers have developed a vaccine that targets OPN5 expression to increase egg production without increasing light.[ref][ref][ref]
It makes sense that light receptors would be involved in thermoregulation in animals and that their response to cold would change during the night versus the day. Keep in mind that animal studies in nocturnal mice may show different results than in diurnal humans.
In humans, OPN5 has been identified in the brain, eyes, testes, fat tissue, and skin.[ref] In the skin, activation of OPN5 increases melanin production in response to UV radiation.[ref]
Genetics, Gene Expression, and Melanopsin:
Genetic variants in the OPN4 gene are associated with seasonal affective disorder, which is a wintertime depression that occurs when light levels are low. The variant is thought to decrease sensitivity to light.
Animal studies show that OPN4 expression in the eye varies over the day. The peak of OPN4 receptor expression is around dusk and dawn. This correlates with its role in setting circadian rhythm, being more sensitive to the changing spectrum of light in the early morning and evening hours.[ref]
Studies show that exposure to intense blue light down-regulates the gene expression for OPN4. A recent study took a more realistic approach and showed that exposure to 150 lux of blue light also decreased gene expression of OPN4 in the retina. For reference 150 lux would be the intensity of standard living room lighting– in this case, lit only with light in the blue wavelength.[ref]
Rare mutations in OPN4 that cause the protein to not function correctly have been shown to cause delayed sleep-wake phase disorder.[ref]
What does light at night do to the body?
I want to put all this information on OPN4 and blue light activation into perspective with a few illustrative studies:
Negatives of bright light at night: Exposure to bright light for two hours before bedtime causes impairments to frontal lobe activation the next day. The results showed that bright light the night before caused a measurable physiological decrease in oxygenation in the brain, which corresponded to a slower decision-making response time.[ref]
Blocking light at night combined with bright morning light: Blocking blue light at night – coupled with bright light for 30 minutes in the morning – has a strong positive effect on brain function in adolescents. The study found increased brain wave activity in the frontal lobe, increased math performance, reduced errors of commission, and enhanced frontal lobe connectivity.[ref]
Bright light in the morning: A study in kidney disease patients (middle-aged adults) showed that exposure to bright light decreased depression symptoms significantly.[ref]
Genotype report:
Access this content:
An active subscription is required to access this content.
Lifehacks: How to optimize your lighting for
It is easy to think of light as harmless and having little effect on health and well-being. I think this is a mistake, and the opsin research shows that we should look at light in the same way we look at exposure to various chemicals.
I would caution that there is still a lot to be discovered on the effects of concentrated light. For example, researchers recently learned that exposing stem cells to green LED light (520 nm) promoted stem cell proliferation by increasing OPN3 expression.[ref] Increasing cell proliferation is a double-edged sword. Research on colon cancer cells shows that certain wavelengths inhibit cell proliferation in laboratory experiments, while others can promote growth.[ref] A study of bacterial growth rates showed that staph grows best under the short blue-green wavelengths from a cell phone.ref]
Artificial light environments:
Access this content:
An active subscription is required to access this content.
Related articles: