Melatonin may actually be the key to health and longevity.
And this is something that I do not say lightly.
This article explores how melatonin is produced and used by the body, as well as its potential role in cancer, aging, and Alzheimer’s disease. It also examines the genes involved in melatonin production and suggests lifehacks to increase its production. Finally, it provides a blueprint for supplementing with melatonin and increasing serotonin and melatonin levels.
How is Melatonin Produced?
Melatonin is a biological molecule found in all vertebrates, insects, cyanobacteria, and plants and is derived from the amino acid tryptophan. Melatonin is categorized as an indoleamine, which refers to the structure of the molecule. (Serotonin is another neurotransmitter that is an indoleamine.)
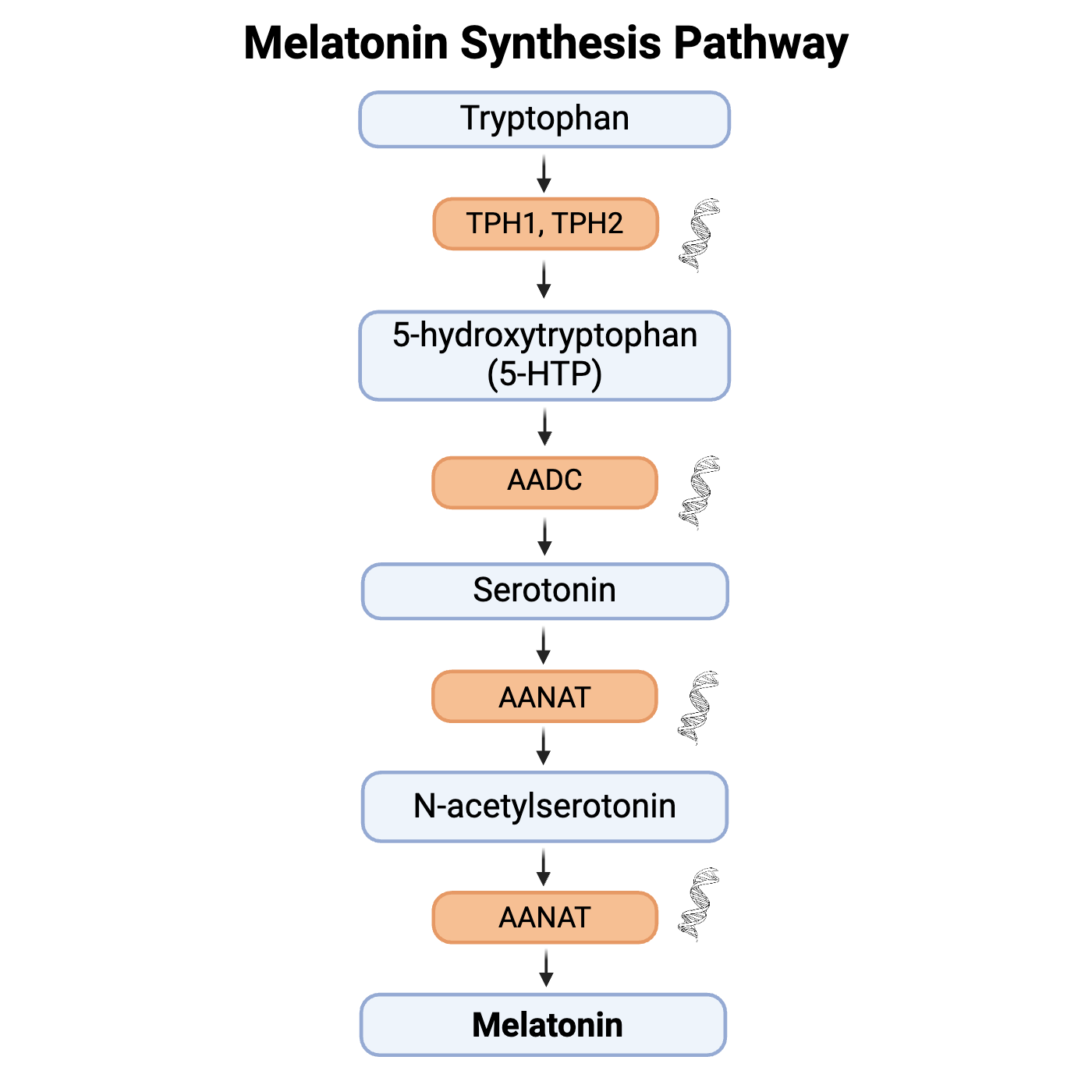
The amino acid tryptophan (found in meats, dairy, and some grains) is converted by the enzyme tryptophan-5-hydroxylase to form 5-hydroxytryptophan, which is then converted to 5-hydroxytryptamine (aka serotonin!).
Serotonin is acetylated to form N-acetylserotonin and then converted to N-acetyl-5-methyltryptamine (aka melatonin!).
Where do you get the tryptophan to begin with? Tryptophan is usually fairly abundant in protein-rich diets.[ref]
Where does all this take place? Both in the brain and inside of cells. You can describe melatonin production in two categories: pineal production and extra-pineal production.
Pineal Gland Production:
The pineal gland is a little organ, about the size of a bean, located in the middle of the brain. It produces and recycles cerebral spinal fluid, similar to the way the kidneys act as a filter for the rest of the body.
The pineal gland produces a bunch of melatonin at night.
Light exposure controls the secretion of melatonin, and the sun going down (lack of light) allows for the rise of melatonin at night.
When light (specifically blue light) hits your eyes in the morning, it triggers the breakdown of melatonin and stops pineal gland production for the day. This is what sets your circadian rhythm – the melatonin from the pineal gland, increasing when the sun goes down and decreasing with the sunrise.[ref]
The cells in the pineal gland contain lots of mitochondria, which researchers think synthesize the melatonin. When light levels fall at sunset, the pineal gland produces melatonin and releases it into both the cerebrospinal fluid and the blood circulation of the brain. The cerebral spinal fluid fills the spaces, or ventricles, surrounding the different regions of the brain.[ref][ref] (I’ll come back to how this impacts brain health and Alzheimer’s disease.)
As you age, the pineal gland will start to calcify, or build up calcium deposits. This happens throughout the aging process, and there are strong associations between the amount of calcification and the neurodegenerative diseases of aging. Thus, the pineal production of melatonin gradually decreases over the course of your life, starting at puberty. The volume of the uncalcified pineal gland correlates to the peak amount of melatonin produced at night.[ref][ref]
Extra-pineal melatonin:
Researchers have found out more recently that melatonin production occurs outside of the pineal gland as well.
Quite a few of your organs and tissues produce melatonin including the gastrointestinal tract, bone marrow, liver, thymus, spleen, heart, skin, testes, placenta, and eggs. Some of the most recent research theorizes that all cells can produce melatonin in their mitochondria.[ref]
Extra-pineal melatonin doesn’t affect the circadian clock or the sleep-wake cycle. Instead, it plays a number of different roles in cells, including as an antioxidant and immune system stimulant.[ref][ref]
Recap: You make melatonin in the brain (pineal gland) at night and it does a bunch of good things in the brain including setting your circadian rhythm. You also make melatonin in the rest of your cells, not just at night, and it acts as an antioxidant within the immune system.
How your body uses melatonin:
First, let’s look at how much melatonin your body makes. The amount depends on age, time of day, and exposure to light. Most studies will measure the peak amount of melatonin produced in the middle of the night. A study of workers (n=117) found that the average peak nighttime melatonin levels for day workers was 15.4 ng/mg creating/hr, and the average peak melatonin for night shift workers was almost 50% less (10.9 ng/mg).[ref] Other sources point to the differences in melatonin due to age. For example, children ages 10 – 15 may produce 120 pg/ml, but adults by age 40 may only be producing 20 – 30 pg/ml.[ref]
Suppression of melatonin by light:
Even very low levels of light (1.5 lux) can suppress melatonin production.
To put this into perspective, a night light puts out about 5 lux, and streetlights outside are around 10 lux. The maximum suppression of melatonin comes at around 305 lux – this would be similar to very bright indoor lighting. So any amount of light over 305 lux doesn’t add to melatonin suppression.
Melatonin Supplement:
The first thing most people think of when it comes to melatonin is using supplemental melatonin for sleep, especially for insomnia. Dosages in melatonin supplements range from 300mcg to 10 mg. An oral dose of 1 to 5 mg will raise serum melatonin levels to 10 to 100 times the normal levels within an hour.[ref]
Taking melatonin during the day increases daytime sleepiness and drops body temperature (something that normally happens around the time you go to bed at night).[ref] Melatonin is easily absorbed orally, subcutaneously, intranasally, transdermally, and sublingually.
Melatonin Receptors:
For melatonin to have a signaling effect on cells, it needs a receptor available on the cell membrane. Two types of melatonin receptors exist, usually referred to as MT1 and MT2 in studies. (More on these receptors in the genetics section below).
The highest density of melatonin receptors (MT1) is in the suprachiasmatic nucleus – or SCN. The SCN is located in the hypothalamus in the brain. It is your body’s center for the circadian pacemaker. This high density of melatonin receptors allows the signals of daytime (lack of melatonin) and nighttime (increased melatonin due to lack of light) to be received in your core circadian clock.[ref]
Circulating melatonin in the bloodstream gets quickly taken up by cells. The cells use it as an antioxidant during times of high oxidative stress. Melatonin can also be transported into the mitochondria or actually made in the mitochondria (the powerhouse of the cell). Melatonin acts as a potent antioxidant to combat the abundant free radicals produced in the mitochondria.[ref]
Studies on Cancer, Aging, and Alzheimer’s:
Cancer:
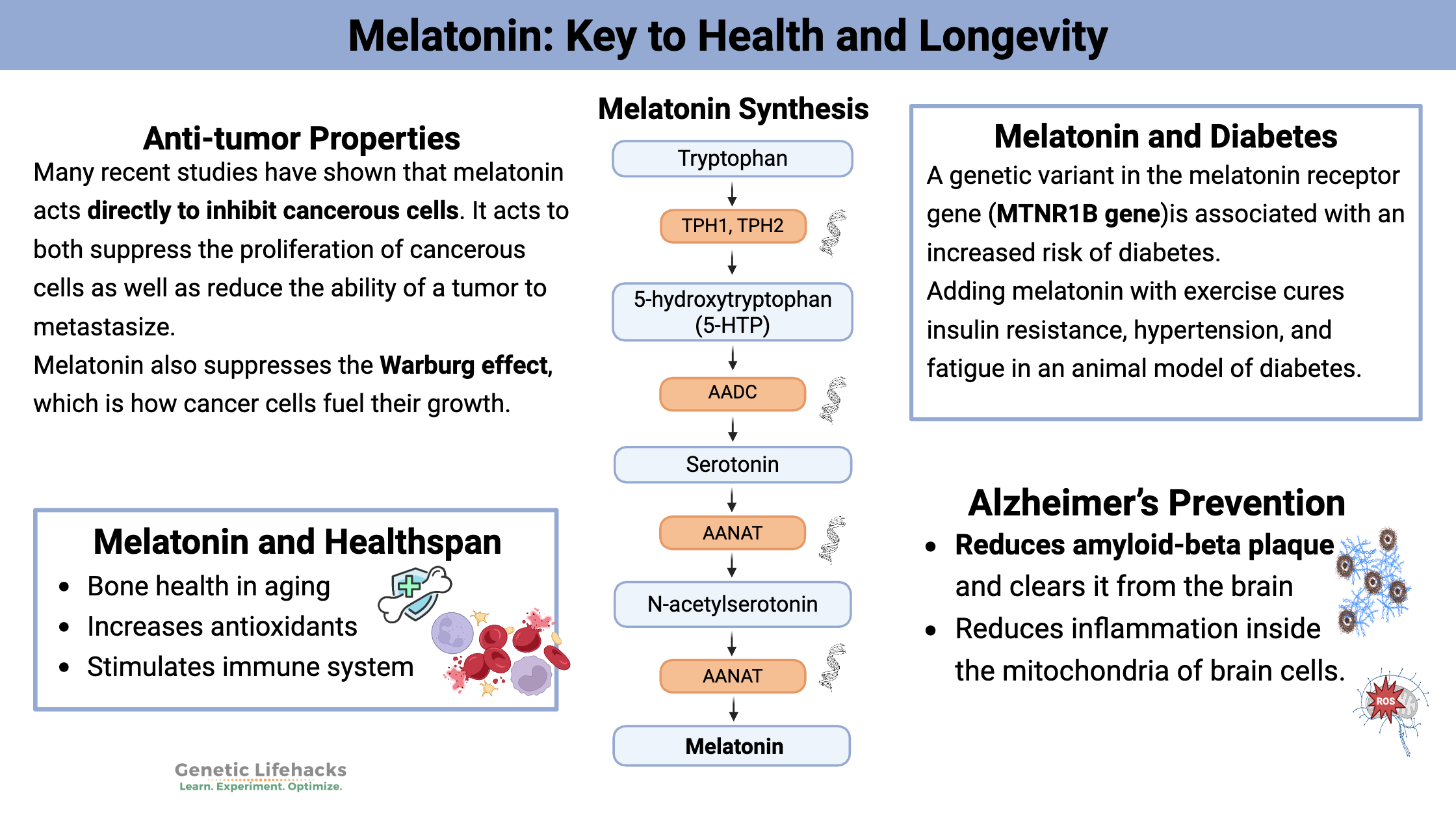
Many recent studies have shown that melatonin acts directly to inhibit cancerous cells. It acts to both suppress the proliferation of cancerous cells as well as reduce the ability of a tumor to metastasize.[ref]
The problem with cancer cells is that they don’t die when they are supposed to. Apoptosis is the process by which cells self-destruct and then are removed. A lot of cancer drugs target this system and increase the apoptosis of both cancerous and normal cells. Melatonin increases apoptosis in cancer cells while being protective in normal cells.[ref]
No, melatonin isn’t as powerful as chemo or a replacement for it. However, it is being used as an adjunct to chemotherapy. It is being successfully used in breast cancer[ref], leukemia[ref], non-small cell lung cancer[ref], colon cancer[ref][ref], head and neck cancer[ref], and more.[ref]
Melatonin also suppresses the Warburg effect, which is how cancer cells fuel their growth. “Under the conditions of exposure to low-intensity light at night and circadian disruption that only suppresses nocturnal melatonin production, both the Warburg effect and the uptake of LA and its metabolism to 13-HODE in breast cancer xenografts become completely arrhythmic and operate at a constitutively high level throughout the entire day (all 24-hrs).”[ref][ref]
Let me point out here that melatonin production decreases with age and cancer rates increase with age…
There is a lot of epidemiological evidence tying cancer rates – especially breast and prostate cancer – to exposure to light at night, which decreases peak melatonin production. Often people dismiss this, thinking there must be another reason for the association between urban lights and cancer. That is a mistake.
The associations between shift work, light at night, and breast cancer have been known for decades. The World Health Organization lists light at night as a probable carcinogen.
The key that ties the epidemiological studies together with an actual mechanism of why light at night causes cancer is that melatonin actively acts as an anti-cancer agent for breast cancer. I encourage you to take a look at the studies on melatonin and breast cancer.[ref][ref][ref][ref] A particularly interesting study looks at the wavelengths of different types of light bulbs in reference to the promotion of cancer growth. (spoiler: LED’s with a lot of blue light promote tumor growth; incandescent bulbs are better)[ref]
Aging: How Melatonin Links to Health Span
What makes you age? What specifically changes as we get older? These are harder questions to answer than you might think. It is something that researchers are still grappling with.
One theory of aging, posited by Harmon in the 1950s, is that it is caused by oxidative stress damaging the cell. The oxidative stress, in part, is caused by reactive oxygen species (ROS) created by the mitochondria. This damage from ROS accumulates and increases with age. Thus, antioxidants (resveratrol, vitamin C, etc) are thought to be anti-aging.
Related article: Resveratrol: Genetic Interactions and Bioavailability
Other researchers, though, think that oxidative stress paints just part of the picture for aging. Another component of aging is the decline in the immune system, known as immunosenescence.[ref][ref]
Melatonin’s role in longevity is thought to be two-part: antioxidant activity and immune system stimulation. The decrease in melatonin as you age parallels the decrease in antioxidant activity and immunosenescence.
In animals, restricting calories has been shown to extend lifespan. It turns out that restricting calories in some animals also preserves the rhythm of melatonin production. Studies also show that giving exogenous melatonin to animals also increases survival time in a similar manner to calorie restriction.[ref] Not all studies show this, so it may be species-specific. Mice studies do show that they live longer when given supplemental melatonin over the course of their lives, starting in adulthood.[ref][ref][ref][ref]
Animal studies also show that melatonin supplementation (beginning in middle to old age) prevents the decrease in bone mass associated with aging.[ref] It also increases mitochondrial function.[ref] Melatonin is necessary to reap the benefits of exercise when aging.[ref] Supplemental melatonin also doubles insulin sensitivity in older animals.[ref]
We don’t do human studies on chronic calorie restriction to extend lifespan – nor have there been long-term studies in humans on supplemental melatonin for fifty-plus years to know the effect. But… there have been interesting studies on specific aspects of anti-aging and healthspan. For example, a study on leukocytes (part of the immune system) shows that melatonin counteracts some of the effects of aging.[ref] Another study points out that foods that have been repeatedly shown to be protective against cardiovascular disease all contain melatonin.[ref]
While I don’t think melatonin is a silver bullet to prevent all aspects of aging, the bulk of the studies do show an overall anti-aging effect.
Alzheimer’s Disease:
Oxidative stress is theorized to be one cause of Alzheimer’s disease, due to free radical damage to the brain. Increased lipid oxidation has been found in autopsied AD brains. The other theorized cause of AD is the accumulation of amyloid-beta plaque in the brain.
Animal studies are quite impressive on the ability of melatonin to reduce amyloid-beta plaque and clear it from the brain. Some studies show the prevention of Alzheimer’s disease and even cognitive enhancement over the control group. The animal models of Alzheimer’s also show that melatonin supplementation increases glutathione and SOD, two important antioxidant enzymes, in the brain. Melatonin supplementation alone, though, wasn’t enough to return the AD mice to normal control levels, but the improvements were significant.[ref][ref][ref][ref]
Another study using a mouse model of AD found that melatonin reduced the signs of Alzheimer’s, including cognitive deficits. The study found that the effect of melatonin was not due to the melatonin receptors but rather acts in a receptor-independent manner to clear amyloid-beta plaque.[ref]
In addition to clearing amyloid-beta plaque, another way that melatonin can protect against Alzheimer’s is through the reduction of ROS in the mitochondria in brain cells.[ref] This may be the key, rather than amyloid-beta clearance. There is uncertainty about whether amyloid-beta accumulation is causing AD vs being present along with AD.
Another theory of Alzheimer’s disease seems it is caused, in part, by altered insulin signaling in the brain. Some researchers are calling it ‘type 3 diabetes’ of the brain. In animal studies, melatonin decreases brain insulin resistance.[ref]
Melatonin levels decrease with aging, while the risk of Alzheimer’s is greatly dependent on age. Studies using constant light to decrease melatonin production show it causes Alzheimer ‘s-like damage in the cells along with increasing the need for antioxidants such as SOD and monoamine oxidase.[ref]
There have been quite a few recent studies and reviews pointing to the role of both low melatonin and circadian disruption in the accumulation of amyloid-beta plaque.[ref]
So what are the results of human studies that have used long-term melatonin supplementation to prevent Alzheimer’s disease? Well, there don’t seem to be any studies on that… Almost all of the studies on Alzheimer’s focus on preventing the disease from getting worse once someone already has been diagnosed with it — rather than stopping AD from happening in the first place.
There are quite a few trials on using melatonin supplements to solve the sleep problems associated with Alzheimer’s. The clinical trials on melatonin range in duration from 10 days to 6 months. They show some positive effects on sleep quality, such as restful sleep, but they don’t show that melatonin supplements reverse the cognitive deficits of AD.[ref]
One placebo-controlled trial of six months of 2mg time-release melatonin in people with mild to moderate AD did show positive results on cognitive performance compared to placebo. The use of melatonin didn’t suddenly cure AD, but it did decrease the loss in cognition.[ref]
Related Article: Alzheimer’s Genes: APOE
Other Studies on Supplemental Melatonin:
Below is an assortment of other studies on melatonin that I found interesting. Bringing them all together helps to make clear the wide-ranging effects of melatonin.
Nerve Regeneration:
An animal study showed melatonin plus chondroitin sulfate ABC helped to regenerate damaged nerves in the C5-C7 region of the neck. Melatonin acts as an anti-inflammatory agent to scavenge free radicals, reduce edema at the injury site, and reduce nerve degeneration. Chondroitin sulfate is the main component of glial scars, and the enzyme chondroitin sulfate ABC reduces it.[ref]
Another animal study used melatonin for six weeks on sciatic nerve injuries. The results showed that melatonin given during the dark period increased the nerve function of the sciatic nerve as well as increased SOD and neural growth factor.[ref]
Skin:
The skin is the largest organ of the body, a protective barrier against the outside world and toxins. Skin cells can synthesize melatonin, and the amount of melatonin depends on ethnic background, gender, and age. African Americans had the highest levels of melatonin. Exposure to UV-B radiation stimulates melatonin, which acts as an antioxidant in the skin. One way it does this is by promoting glutathione production.[ref] And yes, there are companies now producing skin creams that contain melatonin. One side effect is that melatonin increases melanin, thus darkening skin.
Iron overload:
An animal study showed that melatonin decreased the effects of iron overload on bone and stem cell death.[ref] This makes sense because melatonin is a well-known iron chelator.[ref][ref]
Related article: Building Up Iron – Hemochromatosis Mutations
Fertility:
The ovaries and follicle cells contain high levels of melatonin. One aspect of increased infertility due to aging is increased reactive oxygen species within the follicle and oocyte. This decreases egg quality, often leading to infertility problems. Melatonin has been used in clinical trials to increase embryo quality in women undergoing IVF, and it has been used in several studies with women with PCOS to normalize hormones and increase egg quality.[ref][ref][ref][ref] Melatonin also may play a role in keeping the mother’s body from rejecting the fetus.[ref]
Related Article: Genetic links to infertility for women
Gut Microbiome:
It makes sense that melatonin would affect gut microbes since it is both an antioxidant and an immune modulator. Studies show that supplemental melatonin changes the composition of the gut microbiome, reduces weight, decreases fatty liver, and decreases low-grade inflammation.[ref] Another animal study showed that melatonin not only changes the gut microbiome but also improves the intestinal barrier and increases gastrointestinal motility.[ref]
Viral and parasitic infections:
Cardiomyopathy, caused by a viral infection (Coxsackievirus B3), is prevented by supplemental melatonin.[ref] Quite a few parasitic infections show improvement with melatonin including malaria, toxoplasmosis, and trypanosomiasis.[ref] Pretreatment with melatonin decreases the damage to the lungs in RSV infection.[ref] Melatonin may even help with the Ebola virus.[ref]
Migraines:
A randomized placebo-controlled trial showed that melatonin (3mg/night) for 12 weeks was superior to placebo for migraine prevention. It was also slightly better than 25mg/day of amitriptyline (tricyclic antidepressant, commonly prescribed for migraine prevention).[ref]
Related articles: Migraines, genetics, root causes
Inflammation and Obesity:
Low-grade inflammation is associated with obesity. A placebo-controlled trial shows that 6mg of supplemental melatonin decreases inflammatory markers (TNF-alpha, IL-6, and CRP) in obese women.[ref] A meta-analysis of a bunch of studies found that melatonin consistently reduces CRP and IL-6 in people with metabolic syndrome.[ref] Several studies have shown that supplemental melatonin (along with diet) increases weight loss.[ref][ref]
Diabetes:
Adding melatonin with exercise cures insulin resistance, hypertension, and fatigue in an animal model of diabetes.[ref] But what about humans? People with type-2 diabetes have lower peak nighttime melatonin levels. This is important because melatonin acts in the pancreas at night to control insulin release. A genetic variant in the melatonin receptor gene is associated with an increased risk of diabetes (more on that below). The influence of melatonin on diabetes may be directly related to its effect as an antioxidant, as a signaling molecule in the pancreas, and its role in setting the circadian rhythm. Recent studies also show that melatonin may directly influence glucose uptake through the GLUT1 receptor.[ref][ref][ref][ref]
Related article: Diabetes Genes
ADHD and Autism:
There are numerous studies on supplemental melatonin for ADHD. Sleep problems often go hand-in-hand with ADHD for both children and adults. Staying up later while being exposed to light at night suppresses melatonin levels. Taking supplemental melatonin — or just blocking out the light at night — may be helpful to some people with ADHD.
Most trials of melatonin supplementation for ADHD show improvements in sleep and in behavior. A few trials, though, just showed sleep improvement with no effect on behavior.[ref][ref]
A study with 125 autistic children with sleep problems found that melatonin use increased sleep onset time (bedtime) by almost an hour per night and decreased the amount of time that it took to fall asleep. A long-term follow-up trial for the next year found also that participants slept over an hour longer, had better sleep quality, and >50% reduction in wakings.[ref][ref] A meta-analysis of 18 previous studies found that melatonin improved both behavioral traits and increased sleep by 73 minutes.[ref] A study of adults with autism found that patients had decreased peak melatonin secretion compared with a control group.[ref]
Melatonin may be impacting more than just healthy sleep in autism. A recent study on autism found a link to two genetic mutations (not covered by 23andMe) in the gene that codes for the enzyme used to convert serotonin to melatonin. The implication here is that decreased melatonin production due to the genetic variant may be causally involved in autism.[ref]
Related article: ADHD Genes and Root Causes
Irritable Bowel Syndrome:
There have been several clinical trials using melatonin to help with IBS symptoms. Melatonin production within the intestinal tract happens under normal conditions and it is also absorbed from foods. Animal studies show that giving low doses (1-10mcg/kg) of melatonin speeds up intestinal transit times, but higher doses (100-1000mcg/kg) slow transit time significantly.[ref] IBS is often accompanied by increased transit time (diarrhea) or slowed transit time (constipation).
In addition to transit time, melatonin also acts as an anti-inflammatory and an immune system modulator in the intestines. These functions also play a role in melatonin’s effect on IBS.
The clinical studies on melatonin show varied results. Overall, there seems to be a benefit of decreased abdominal pain and regulated transit time. Almost half of IBS patients taking melatonin showed improved quality of life, which was significantly higher than those taking a placebo.[ref]
Related article: IBS Genes
Melatonin Genotype Report:
Not a member? Join here. Membership lets you see your data right in each article and also gives you access to the member’s only information in the Lifehacks sections.
There are several ways that your genes interact with melatonin: through production, cellular receptors, and metabolism. There are not a lot of genetic variants, though, that significantly impact the production of melatonin. This may be because it is vital to so many processes in the body. Below is an overview of some of the studies associated with melatonin-related genetic variants that are available in 23andMe or AncestryDNA data.
MTNR1B gene:
This gene codes for the MT2 melatonin receptor.
The rs1030963 variant is very well studied for its impact on overnight insulin release and glucose levels. It has links to an increase in the risk of diabetes and gestational diabetes.[ref][ref][ref][ref] A reduction in risk for diabetes to back-to-normal risk allele carriers occurs if they eat dinner earlier.[ref]
Check your genetic data for rs10830963 (23andMe v4, v5 ; AncestryDNA):
- G/G: linked to a higher risk of diabetes, increased fasting glucose – don’t eat dinner late
- C/G: linked to a higher risk of diabetes, increased fasting glucose – don’t eat dinner late
- C/C: typical
Members: Your genotype for rs10830963 is —.
Check your genetic data for rs1387153 (23andMe v4, v5, AncestryDNA):
- T/T: increased fasting glucose, increased risk of gestational diabetes.[ref]
- C/T: somewhat increased fasting glucose
- C/C: typical
Members: Your genotype for rs1387153 is —.
MTNR1A gene:
This gene codes for the MT1 melatonin receptor.
Check your genetic data for rs2375801 (23andMe v4)
- C/C: increased risk of cancer metastasis (liver)[ref]
- C/T: increased risk of cancer metastasis (liver)
- T/T: typical
Members: Your genotype for rs2375801 is —.
Check your genetic data for rs6553010 (23andMe v4, AncestryDNA):
- A/A: increased risk of cancer metastasis (liver)[ref]
- A/G: increased risk of cancer metastasis (liver)
- G/G: typical
Members: Your genotype for rs6553010 is —.
Check your genetic data for rs12506228 (23andMe v4, v5; AncestryDNA):
- A/A: likely fewer melatonin receptors in the brain, a greater impact from working the night shift, increased risk of Alzheimer’s.[ref][ref]
- A/C: somewhat fewer melatonin receptors, somewhat impacted from light at night, increased risk of Alzheimer’s
- C/C: typical MTNR1A variant
Members: Your genotype for rs12506228 is —.
AANAT gene:
The alkylamine N-acetyltransferase (AANAT) gene controls the rhythmic production of melatonin by the pineal gland. The AANAT variant rs28936679 is a rare variant that causes delayed phase sleep disorder.[ref]
Check your genetic data for rs28936679 (23andMe v4 only):
- A/G: may cause delayed phase sleep disorder
- G/G: typical
Members: Your genotype for rs28936679 is —.
TPH2 Gene:
The TPH2 gene codes for the rate-limiting enzyme involved in the conversion of tryptophan to serotonin, which can then be converted to melatonin. The link between TPH2 and melatonin levels is indirect, but I’ve included it here to illustrate the connection between melatonin precursors and disrupted circadian rhythm.[ref][ref]
Check your genetic data for rs4290270 (23andMe v4; AncestryDNA):
- T/T: increased risk of waking early, increased risk of depression[ref][ref]
- A/T: probably a slightly increased risk of waking early, depression (this is the most common genotype)
- A/A: typical
Members: Your genotype for rs4290270 is —.
Check your genetic data for rs4570625 (23andMe v4, v5; AncestryDNA):
- G/G: (common genotype) less TPH2 function[ref], more likely to achieve depression remission with escitalopram*[ref]
- G/T: higher TPH2
- T/T: higher TPH2, less depression[ref]
Members: Your genotype for rs4570625 is —.
*Escitalopram is an antidepressant that acts on the circadian system.[ref]
Methylation cycle:
In the process of turning serotonin into melatonin, your body uses a methyl group. There are quite a few genetic variants that decrease the production of methyl groups, including MTHFR variants. I don’t have any studies, though, that show genetic variants that impact methylation also decrease melatonin.
Lifehacks:
This members-only section includes additional lifehacks including supplement research. Additionally, you will see your genetic data matched to specific recommendations. Consider joining today to see the rest of this article.
Access this content:
An active subscription is required to access this content.
Conclusion:
Melatonin is important to your body in many ways. It is produced in high levels at night in the pineal gland, setting your circadian rhythm and providing vital anti-inflammatory and immune functionality to the brain at night. This nighttime production of pineal melatonin is based on a lack of light hitting the retina. Melatonin is also produced both day and night at low levels in tissues throughout the body, countering ROS in the mitochondria and providing other benefits throughout the body.
As an antioxidant, melatonin works to promote healthy aging, prevent Alzheimer’s, fight cancer, and improve many other chronic conditions. The circadian mismatch of artificial light at night (and lack of sunlight exposure during the day) may be reducing melatonin levels and increasing the risk of these chronic conditions.
Related Articles and Genes:
Bipolar disorder, depression, and circadian clock genes
New research shows depression and bipolar disorder are linked with changes or disruptions in circadian genes. Also, genetic variants in the circadian clock genes can increase your susceptibility to mood disorders.
Tryptophan: A building block for serotonin and melatonin
Tryptophan is an amino acid that the body uses to make serotonin and melatonin. Genetic variants can impact the amount of tryptophan that is used for serotonin. This can influence mood, sleep, neurotransmitters, and immune response.
Alzheimer’s and APOE genotype
The APOE gene variants are tightly linked with the risk of Alzheimer’s disease. Find out whether you carry the APOE risk type for Alzheimer’s – and learn what we can do via diet and lifestyle to prevent this disease.
Seasonal Affective Disorder:
Find out how circadian rhythm genes are linked to seasonal depression, and discover which solutions may work best for you.
References:
A, Sookprasert, et al. “Melatonin in Patients with Cancer Receiving Chemotherapy: A Randomized, Double-Blind, Placebo-Controlled Trial.” Anticancer Research, vol. 34, no. 12, Dec. 2014. pubmed.ncbi.nlm.nih.gov, https://pubmed.ncbi.nlm.nih.gov/25503168/.
B. Arnao, Marino, and Josefa Hernández-Ruiz. “The Potential of Phytomelatonin as a Nutraceutical.” Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry, vol. 23, no. 1, Jan. 2018, p. 238. PubMed Central, https://doi.org/10.3390/molecules23010238.
Cj, Shen, et al. “Melatonin Suppresses the Growth of Ovarian Cancer Cell Lines (OVCAR-429 and PA-1) and Potentiates the Effect of G1 Arrest by Targeting CDKs.” International Journal of Molecular Sciences, vol. 17, no. 2, Jan. 2016. pubmed.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms17020176.
Dollins, A. B., et al. “Effect of Inducing Nocturnal Serum Melatonin in Daytime on Sleep, Mood, Body Temperature, And.” Proceedings of the National Academy of Sciences of the United States of America, vol. 91, no. 5, Mar. 1994, pp. 1824–28. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC43256/.
Grivas, Theodoros B., and Olga D. Savvidou. “Melatonin the ‘Light of Night’ in Human Biology and Adolescent Idiopathic Scoliosis.” Scoliosis, vol. 2, Apr. 2007, p. 6. PubMed Central, https://doi.org/10.1186/1748-7161-2-6.
J, Wu, et al. “Light at Night Activates IGF-1R/PDK1 Signaling and Accelerates Tumor Growth in Human Breast Cancer Xenografts.” Cancer Research, vol. 71, no. 7, Apr. 2011. pubmed.ncbi.nlm.nih.gov, https://doi.org/10.1158/0008-5472.CAN-10-3837.
Konturek, S. J., et al. “Role of Melatonin in Upper Gastrointestinal Tract.” Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society, vol. 58 Suppl 6, Dec. 2007, pp. 23–52.
Liebrich, Luisa-Sophie, et al. “Morphology and Function: MR Pineal Volume and Melatonin Level in Human Saliva Are Correlated.” Journal of Magnetic Resonance Imaging: JMRI, vol. 40, no. 4, Oct. 2014, pp. 966–71. PubMed, https://doi.org/10.1002/jmri.24449.
M, Fic, et al. “The Impact of Melatonin on Colon Cancer Cells’ Resistance to Doxorubicin in an in Vitro Study.” International Journal of Molecular Sciences, vol. 18, no. 7, June 2017. pubmed.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms18071396.
N, Rybnikova, et al. “Kernel Density Analysis Reveals a Halo Pattern of Breast Cancer Incidence in Connecticut.” Spatial and Spatio-Temporal Epidemiology, vol. 26, Aug. 2018. pubmed.ncbi.nlm.nih.gov, https://doi.org/10.1016/j.sste.2018.06.003.
Onseng, Kittipong, et al. “Beneficial Effects of Adjuvant Melatonin in Minimizing Oral Mucositis Complications in Head and Neck Cancer Patients Receiving Concurrent Chemoradiation.” Journal of Alternative and Complementary Medicine (New York, N.Y.), vol. 23, no. 12, Dec. 2017, pp. 957–63. PubMed, https://doi.org/10.1089/acm.2017.0081.
Papantoniou, Kyriaki, et al. “Circadian Variation of Melatonin, Light Exposure, and Diurnal Preference in Day and Night Shift Workers of Both Sexes.” Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, vol. 23, no. 7, July 2014, pp. 1176–86. PubMed, https://doi.org/10.1158/1055-9965.EPI-13-1271.
Pires-Lapa, Marco A., et al. “β-Adrenoceptors Trigger Melatonin Synthesis in Phagocytes.” International Journal of Molecular Sciences, vol. 19, no. 8, July 2018, p. 2182. PubMed Central, https://doi.org/10.3390/ijms19082182.
Q, Wang, et al. “Melatonin Sensitizes Human Colorectal Cancer Cells to γ-Ray Ionizing Radiation In Vitro and In Vivo.” International Journal of Molecular Sciences, vol. 19, no. 12, Dec. 2018. pubmed.ncbi.nlm.nih.gov, https://doi.org/10.3390/ijms19123974.
Reiter, R. J. “The Ageing Pineal Gland and Its Physiological Consequences.” BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, vol. 14, no. 3, Mar. 1992, pp. 169–75. PubMed, https://doi.org/10.1002/bies.950140307.
Reiter, Russel J., et al. “Melatonin as a Mitochondria-Targeted Antioxidant: One of Evolution’s Best Ideas.” Cellular and Molecular Life Sciences: CMLS, vol. 74, no. 21, Nov. 2017, pp. 3863–81. PubMed, https://doi.org/10.1007/s00018-017-2609-7.
Sm, Hill, et al. “Melatonin: An Inhibitor of Breast Cancer.” Endocrine-Related Cancer, vol. 22, no. 3, June 2015. pubmed.ncbi.nlm.nih.gov, https://doi.org/10.1530/ERC-15-0030.
Suofu, Yalikun, et al. “Dual Role of Mitochondria in Producing Melatonin and Driving GPCR Signaling to Block Cytochrome c Release.” Proceedings of the National Academy of Sciences of the United States of America, vol. 114, no. 38, Sept. 2017, pp. E7997–8006. PubMed, https://doi.org/10.1073/pnas.1705768114.
Tan, Dun Xian, et al. “Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland.” Molecules : A Journal of Synthetic Chemistry and Natural Product Chemistry, vol. 23, no. 2, Jan. 2018, p. 301. PubMed Central, https://doi.org/10.3390/molecules23020301.
Zhang, Jiao-Jiao, et al. “Effects of Melatonin on Liver Injuries and Diseases.” International Journal of Molecular Sciences, vol. 18, no. 4, Mar. 2017, p. 673. PubMed Central, https://doi.org/10.3390/ijms18040673.