Key takeaways:
~ The MTHFR gene impacts how your body utilizes folate (vitamin B9) for creating neurotransmitters, detoxifying toxicants, and maintaining a healthy heart.
~ Genetic variants can impact how well this gene works.
~ Optimizing diet and supplements related to methylation may impact several aspects of wellness.
Check your raw data for C677T, A1298C, and more
This article shows you what to check in your raw data, then explains the scientific research on the MTHFR variants. At the end of the article, you will find solid, evidence-based lifestyle solutions for optimizing the MTHFR variants.
Frankly, there is a lot of misinformation on the internet about the MTHFR gene mutations, so I will explain the peer-reviewed research studies here.
I’ll clearly explain how to optimize your diet (or add in supplements) if you carry MTHFR genetic variants.
MTHFR Gene Mutations Genotype Report:
Let’s put the rest of this article into context by checking your two more common MTHFR variants first. (I’ll include other MTHFR variants later in the article).
You don’t need to do specific genetic testing or buy health reports for the MTHFR variants. The information is already included in your raw data from 23 and Me or AncestryDNA.
Not a member? Join Here.
Membership lets you see your genotype in articles and gives you access to the members-only information in the Lifehacks sections.
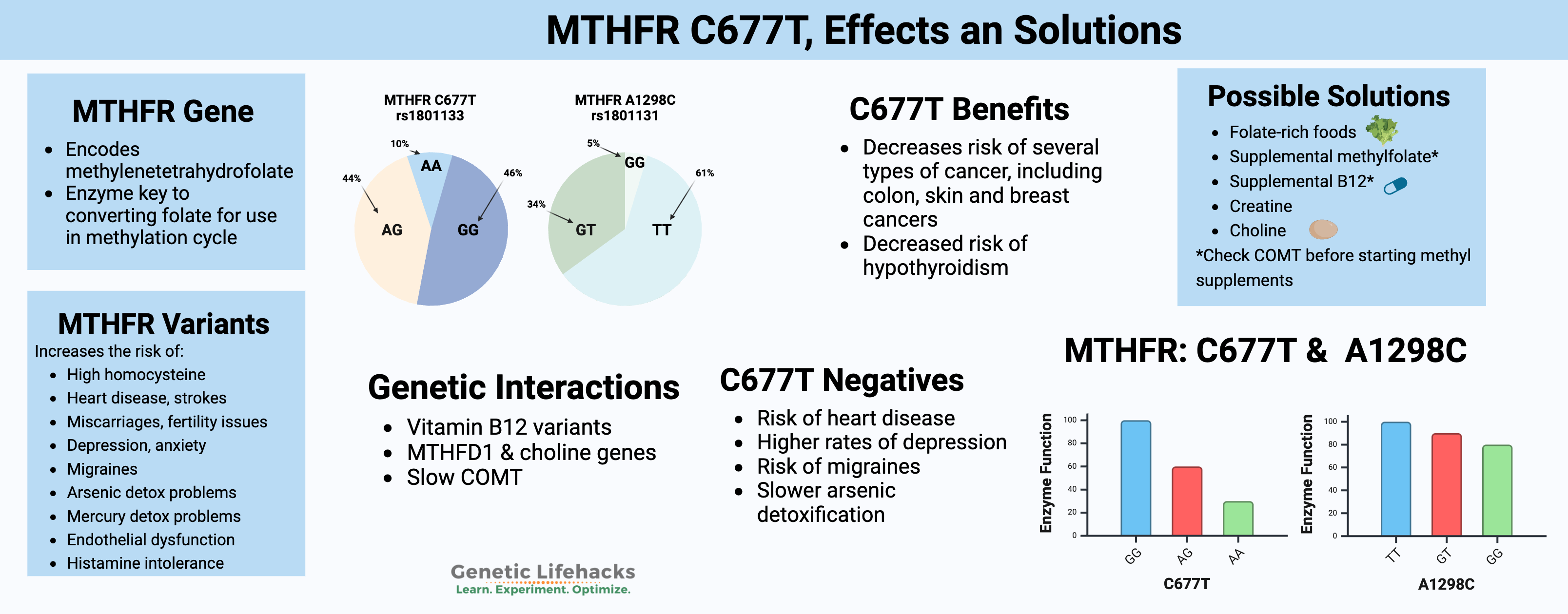
Check your genetic data for rs1801133 (23andMe v4, v5; AncestryDNA):
- G/G: typical *
- A/G: one copy of MTHFR C677T allele, enzyme function decreased by 40%
- A/A: two copies of MTHFR C677T, enzyme function decreased by 70 – 80%
Members: Your genotype for rs1801133 is —.
Check your genetic data for rs1801131 (23andMe v4, v5; AncestryDNA):
- T/T: typical *
- G/T: one copy of MTHFR A1298C (heterozygous), slightly decreased enzyme function
- G/G: two copies of MTHFR A1298C (homozygous), decreased enzyme by about 20%
Members: Your genotype for rs1801131 is —.
Let’s dig into the details of what “MTHFR” is and what the research shows about these variants.
What is the MTHFR gene?
The MTHFR gene codes for an enzyme that is an important part of the methylation cycle. The enzyme is called methylenetetrahydrofolate reductase or MTHFR (same as the gene).
There is a lot of swirl about MTHFR — with people thinking the “MTHFR mutation” is the cause of everything under the sun. It seems to have caused a backlash, with doctors claiming the variants are completely unimportant.
Let’s cut through the hype, and stick with the research:
Essentially, research shows that the MTHFR variants statistically increase the risk of quite a few health issues… But this relative risk needs to be kept in perspective and considered along with your diet and exposure to toxins.
What is the methylation cycle?
I mentioned above that the MTHFR gene codes for an enzyme that is an important part of the methylation cycle.
Methylation is the adding and removing of a methyl group (CH3) to amino acids, DNA, and other enzymes or proteins.
Forming new molecules:
Most of the molecules in our body are chains of hydrocarbons — carbons plus hydrogens. So adding a methyl group stacks on one more link in a hydrocarbon chain. Sometimes it helps me to visualize it as the molecule being made of Legos, and the methyl group is just adding another Lego component to your creation.
Adding a methyl group – or an extra carbon plus three hydrogens – then changes the original molecule into something different.
Let’s take melatonin as an example: A methyl group is added to serotonin to convert it to melatonin.
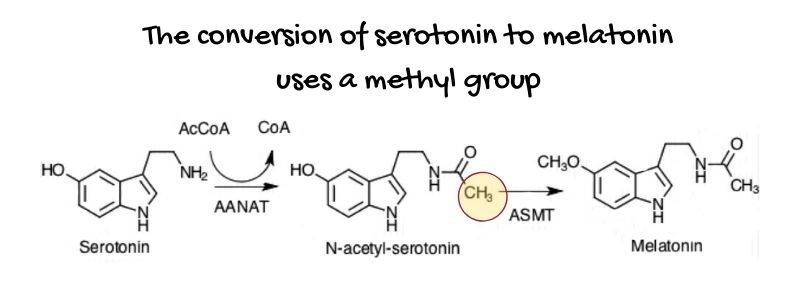
DNA methylation:
A methyl group is also vital for your DNA in the cell nucleus. By binding to specific spots on a chromosome, methylation can turn on and off genes, maintain and repair your DNA, and alter proteins.
Detoxification and breaking down substances:
Methylation is essential in the nervous system, in the production and breakdown of neurotransmitters, and in detoxifying some environmental toxicants.
Homocysteine:
The methylation cycle also intimately impacts heart health. It controls the level of homocysteine, an important marker of heart disease risk. It is also involved in cholesterol levels.[ref] Genetic variants in the methylation pathway have been linked to high homocysteine levels and heart disease in many studies.
Hormones:
Additionally, the pathway involves the regulation of hormones, such as estrogen, as well as playing a role in histamine levels.
Related article: Histamine metabolism and estrogen receptors
As you can see, optimizing your methylation cycle can balance many health issues.
So how does the body make methyl groups? That is where MTHFR comes in.
What does the MTHFR enzyme do?
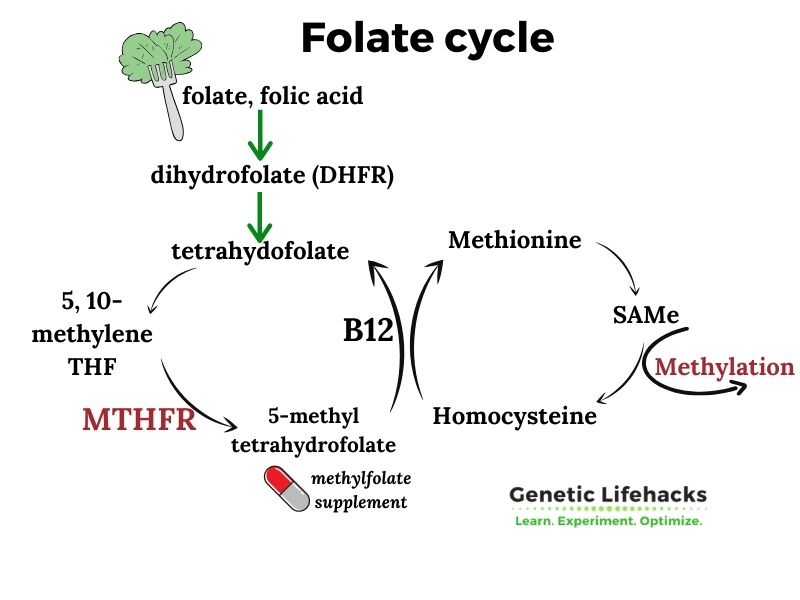
MTHFR is a central gene in the methylation cycle and is a limiting factor for producing methyl groups from folate (vitamin B9). Common genetic variants in the coding of this gene affect more than half the population.
Specifically, the MTHFR gene codes for an enzyme called methylenetetrahydrofolate reductase that turns folate into the active form, 5-methyltetrahydrofolate, that your body uses.
This enzyme, along with the active form of vitamin B-12 (methylcobalamin), drives an essential portion of the methylation cycle. While folate is important, I’ll also explain in the Lifehacks section how choline can also be a source of methyl groups…
What do C677T and A1298C mean? Getting the terms right…
Let’s talk about terminology…
- A mutation is a change in a gene that happens in less than 1% of the population. These are rare changes.
- A polymorphism is a change that occurs in the gene for more than 1% of the population. We all have lots of different polymorphisms — these are the small changes that make us all unique.
The MTHFR C677T variant is considered a single nucleotide polymorphism – a SNP. One nucleotide base pair (the As, Cs, Gs, and Ts) is different for part of the population.
By genetic definitions, MTHFR C677T and A1298C are NOT mutations. Both are common variants, or SNPs. And both polymorphisms are found in about half the population.
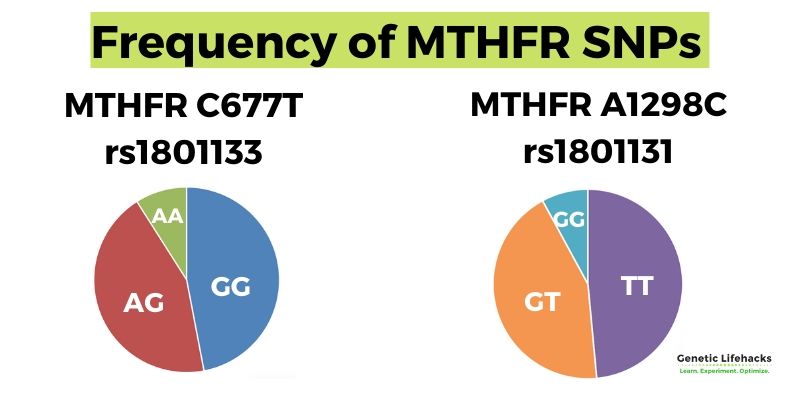
Conditions linked to the MTHFR C677T or A1298C variants
While these variants are common, they do impact health and wellness — especially for people who don’t eat a lot of foods high in folates, such as dark leafy greens or beef liver.
The MTHFR gene is one of the most well-researched genes, with over 6,000 studies investigating the common C677T variant.
Studies show that the C667T and A1298C variants increase the risk of numerous health conditions:
Important: Carrying an MTHFR variant increases the relative risk of many chronic diseases, but this does not mean it will cause you to have that disease. Simply put, these variants increase susceptibility to diseases when diet and lifestyle factors are not optimal.
Importantly, food choices or supplemental methylfolate can eliminate the problems from the MTHFR variants.
Depression and the MTHFR Gene Variants:
Let me go a little more in-depth here on the research that shows that MTHFR variants increase susceptibility to mood disorders.
- A meta-analysis, which included 26 different published studies, found that the MTHFR C677T variant was associated with an increased risk of depression in people with different ancestry groups.[ref]
- Age and gender may also play a role here. Postmenopausal women who carried the C677T variant had a 2 to 3-fold increased risk of depression. The study population group was Polish women.[ref]
- Women who were homozygous (two copies) for the A1298C variant were at twice the risk of major depressive disorder (MDD). The risk of MDD was even higher in COMT slow (MET) allele carriers. (read about COMT)[ref]
I do want to point out that not all studies agree, and some researchers found that the MTHFR C677T variant has little to no impact on depression risk. The difference may be due to the role that diet plays here. People who eat a diet that includes more folate (green veggies, legumes, etc.) may not be at an increased risk of mood disorders, while people who are folate-deficient may be more susceptible to depression.[ref][ref]
Related article: Getting to the root cause of depression
Getting specific on the MTHFR C677T variant:
The C677T SNP is a change in one nucleotide base at one spot in the gene. The variant slightly changes the protein structure, which causes the enzyme to break down faster at normal body temperature. Faster enzyme breakdown reduces the amount of enzyme available.[ref][ref][ref]
People with the rs1801133 A/A genotype have the greatest impact. They have about a 70% reduction in enzyme function.
Combined with a diet lower in folate, this more severe reduction in enzyme function impacts many aspects of health.
For example, recent research studies show:
- In cancer patients treated with 5-FU, neutropenia is 7-fold more likely with C677T.[ref]
- In epileptic patients, supplemental folate and B12 decreased seizure frequency in C677T carriers.[ref]
- In women with two copies of C677T (AA genotype), average blood pressure was higher by 10 mmHg for systolic BP.[ref]
- The C677T variant increases the relative risk of autism a little (33-66%). Keep in mind that this is an increase in relative risk, and other environmental and genetic factors are also involved in autism.[ref]
Why does the MTHFR variant increase the risk of heart disease?
Extensive studies are showing a link between MTHFR C677T and an increased risk of cardiovascular disease.
A meta-study that combined other study results shows that two copies of the MTHFR C677T variant (A/A genotype) increase the relative risk of heart disease by 38%.[ref] With heart disease being the number one killer in most countries, a 38% increase in risk is important.
The increased risk of heart disease is often explained as being due to high homocysteine levels in people with the MTHFR variant who don’t get adequate folate.[ref] High homocysteine levels have long been a marker of increased risk of heart disease.
A new research study, though, shows that people with MTHFR C677T variants (AG or AA) have reduced endothelial function, even when homocysteine levels are normalized by increasing folate intake.[ref] The endothelium is the lining of blood vessels, and endothelial function controls how the blood vessels relax or contract to control blood pressure. Additionally, endothelium function involves the release of the right enzymes to control blood clotting, immune function, and platelet stickiness. The study showed, using a mouse model of MTHFR C67T, that SIRT1 was down-regulated even when homocysteine was normalized. This affected clotting and endothelial function, and supplemental resveratrol helped to restore endothelial function.
Related article: Resveratrol: Genetic Interactions and Bioavailability
Overall, optimizing your methylation cycle can be important to heart health. Take the knowledge of carrying a C677T variant as a ‘heads up’ to pay attention to your heart and be proactive about your health.
Related article: MTHFR, riboflavin, and reducing high blood pressure
Migraines:
Numerous studies show that MTHFR variants are linked to a significantly increased risk of migraines.
Some studies indicate that the risk is also due to higher homocysteine levels[ref], while other studies show that it may be due to the methylation of certain genes.[ref]
Looking at the data:
- Meta-analyses showed that the MTHFR C677T variant increased the risk of migraines with aura for all populations. In non-Caucasians, the increased risk was a whopping 3-fold.[ref][ref]
- A study examining the electrophysiological characteristics of migraines found that carriers of the MTHFR C677T variant were more likely to get migraines and more likely to have photophobia with migraines.[ref]
- The A1298C variant was associated with the risk of migraines in a North Indian population.[ref]
Related article: Getting to the root genetic cause of migraines
Detoxification and MTHFR: heavy metals and more
Another way methyl groups are necessary to health is by detoxifying toxicants. The body has many different means of rendering toxicants water-soluble and able to be excreted. Some of these involve the addition of methyl groups.
For example, a methyl group is needed in the detoxification reaction for arsenic. The enzyme (arsenite methyltransferase) that metabolizes arsenic depends on the availability of methyl groups. The C677T variant is linked to decreased arsenic detoxification and increased skin lesions with exposure.[ref][ref]
Related article: Arsenic detoxification genes
Additionally, methylation is important in detoxifying mercury. In fact, MTHFR variants are tentatively linked to being more likely to have problems detoxifying mercury (small study).[ref][ref]
Related article: Mercury detoxification genes
Tradeoffs! Balancing negative effects with the positive benefits of MTHFR C677T
You may wonder why MTHFR variants with such negative effects are so common in all population groups. It seems like a variant that should have been weeded out with natural selection. One thing I’ve noticed is that for common negative variants, there is almost always a positive effect that balances it out.
The big positive for MTHFR C677T is that it protects against several common types of cancer.
Studies finding protective effects:
- A meta-analysis found that two copies of the C677T variant (AA genotype) decrease the risk of colon cancer by about 20%![ref]
- Another meta-analysis found that two copies of the C677T variant (AA genotype) were protective against prostate cancer.[ref]
- Having either one or two copies of MTHFR C677T is protective against susceptibility to retinoblastoma[ref] and oral squamous cell cancer.[ref]
- A longevity study in China found that the T allele was more prevalent among their long-lived group (aged 90 and older), especially in females.[ref]
- Another Chinese study found two copies of the C677T variant (AA genotype) were protective against gastric cancer.[ref]
Why does decreasing methyl groups protect against cancers in the colon or stomach? The methyl groups are needed to support the excessive and quick growth of tumors, so limiting them decreases the replication of cells, including cancer cells. In fact, methotrexate was one of the first chemotherapy drugs discovered. Methotrexate is an ‘antifolate’ that works by inhibiting the conversion of dietary folate into methylfolate. The lack of methylfolate then inhibits the excessive cellular growth of tumors.[ref]
Studies finding protective effects of MTHFR A1298C:
The MTHFR A1298C variant (GG or GT genotypes) is associated with a decreased risk of hypothyroidism.[ref]
Why do some doctors think that MTHFR variants are not important?
The very extensive research (it’s one of the most researched genes) shows MTHFR variants have links to an increased relative risk for the disorders listed above and many other diseases.
While an MTHFR variant can play a role in susceptibility to many chronic diseases, it usually is not the absolute cause of most diseases. Instead, it’s just one part of the picture.
When patients flood into the doctor’s office to demand treatment for ”having the MTHFR gene”, the automatic backlash is to say that MTHFR variants aren’t important. (Terminology pet peeve – everyone has the MTHFR gene! It’s one of those genes you can’t live without.)
Most physicians are trained to treat diseases or symptoms rather than hunting down the various aspects of lifestyle, diet, and environment that could contribute to the disease. No one has the time in a 10-minute visit to discuss the nuances of diet, genetics, toxicant exposure, etc. To be fair, most people going to a doctor with an ailment expect to get a simple answer or a pill that solves the problem immediately.
Digging deeper: More than just C677T and A1298C
While the C677T and A1298C are the most well-studied variants, there are several other genetic variants in the MTHFR gene that either increase or decrease the enzyme’s function.
Hey! Want to go further with your genetic raw data? Join as a Member!
Additional MTHFR Genotype Report:
Variants that decrease MTHFR enzyme function:
In addition to the MTHFR C677T and A1298C variants, the G1793A variant also significantly decreases enzyme function.
Check your genetic data for rs2274976 G1793A or R594Q (23andMe v4, v5; AncestryDNA):
- T/T: associated with cleft lip[ref], neural tube defect[ref], higher homocysteine, and folate deficiency[ref], increased risk of schizophrenia in children[ref][ref], cognitive issues possible for seniors with this genotype in conjunction with low vitamin B12[ref], risk of lower bone mineral density if B12 is also low[ref]
- C/T: somewhat increased risk of schizophrenia, cognitive issues possible for seniors with this genotype in conjunction with low vitamin B12[ref]
- C/C: typical
Members: Your genotype for rs2274976 is —.
Variants that are associated with positive outcomes:
Check your genetic data for rs9651118 (23andMe v4; AncestryDNA):
- T/T: most common genotype
- C/T: decreased risk of liver cancer[ref], slower cognitive decline in the elderly[ref], lower homocysteine, type 2 diabetes (compared to T/T)[ref][ref]
- C/C: decreased risk of lung cancer[ref], lower homocysteine, type 2 diabetes (compared to T/T)[ref][ref]
Members: Your genotype for rs9651118 is —.
Check your genetic data for rs13306560 (23andMe v4; AncestryDNA):
- T/T: avg 5.2 mmHg lower diastolic blood pressure[ref], protective against Parkinson’s[ref]
- C/T: avg 2.6 mmHg lower diastolic blood pressure
- C/C: typical
Members: Your genotype for rs13306560 is —.
Check your genetic data for rs17367504 (23andMe v4, v5; AncestryDNA):
- G/G: protective against hypertension, preeclampsia[ref]
- A/G: protective against hypertension, preeclampsia
- A/A: typical
Members: Your genotype for rs17367504 is —.
Lifehacks: Actions you can take with MTHFR
Knowing that you carry an MTHFR genetic variant can help guide your choice of foods and supplements. By optimizing your diet, you can mitigate the risks from the MTHFR variants.[ref]
Diet for MTHFR:
Increase folate-rich foods:
Increasing your intake of folate (vitamin B9) from foods will help mitigate some of the risks from the MTHFR variant. Foods containing lots of folates include leafy greens, lentils, liver, asparagus, liver, and broccoli.
Is dietary folate enough?
A recent study showed that increasing folate-rich foods reduced homocysteine levels and inflammatory markers in women with the MTHFR C677T variant. The study showed that eating 191 mcgs of folate from vegetables each day caused a significant beneficial change.[ref]
Need recipe ideas? Check out Folate-rich foods and recipes for MTHFR
Folate is not exactly folic acid…
Note that when looking at folate content, you need to ensure the ingredients list doesn’t refer to folic acid (a synthetic form found in processed foods). Not everyone processes folic acid the same way.
Related article: Folic acid and your DHFR gene
Increase your choline intake:
Choline can help your body bypass a lack of folate in the methylation cycle.[ref][ref]
Good sources of choline include egg yolks, beef liver, and wheat germ.
A metabolite of choline, betaine, is what works through the methylation cycle; therefore, food sources of betaine (beets, quinoa, and spinach) are also helpful here. Supplemental betaine (also called TMG) is also available. (People with two copies of the slow COMT variant may want to be careful with supplementing with TMG.)
Related article: Choline-rich foods and recipe ideas
MTHFR Supplements:
If your diet doesn’t provide enough nutrients needed in the methylation cycle, you may want to consider supplements to increase your intake. Alternatively, you could experiment with supplements on a short-term basis to see exactly which nutrients you need to include more of in your diet.
Supplemental methylfolate and B12:
- If you aren’t getting enough folate from foods, you could try a low-dose methylfolate supplement. Methylfolate is the active form of folate (vitamin B9).
- Vitamin B12 is also important in the methylation cycle, so you need to ensure you are getting enough B12 either through diet (animal-based foods) or supplements.
Note: People with the slow COMT genetic variant may want to be careful about high-dose supplements that affect the methylation cycle, such as methylfolate and methylB12. Instead, stick with dietary folate and use alternative forms of B12, such as adenosyl and hydroxocobalamin. Read all about COMT and supplement interactions.
The rest of this article goes into details on supplements and other genetic variants to consider along with MTHFR. It is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
High Histamine and Methylation (MTHFR variants)
Histamine is a molecule that plays many roles in the body. It is involved in allergic reactions, plays a role in our immune defense system, acts as a vasodilator, and is a neurotransmitter. While most of us think of histamine only when reaching for an antihistamine during allergy season, it is a vital part of our body’s everyday functions.
Spike Protein, Mast Cells, and Histamine
Do you know of someone with unexplained heart palpitations, spiking blood pressure, dizziness, and tinnitus? Discover how research is linking these symptoms to histamine, mast cells, and the spike protein.
Fibrinogen: Blood clot risk factor
Fibrinogen is a protein that is essential for creating blood clots when you get a wound. But higher levels of fibrinogen are a major risk factor for heart disease and DVT. Learn how your genes impact your fibrinogen level.
MTHFR and Vaccinations
You may have read or heard that anyone who carries MTHFR variants should not be vaccinated. Usually, the reason given is that those with decreased MTHFR enzyme activity cannot detoxify or ‘handle’ vaccinations, often with references to mercury in the vaccines. This article reviews the published scientific studies on the topic of MTHFR and vaccinations.
Vitamin B12 genes
MTR (methionine synthase) and MTRR (methionine synthase reductase) are two genes involved in the conversion of homocysteine to methionine, and a couple of fairly common gene variants cause the genes to work differently.
References:
Abhinand, P. A., Shaikh, F., Bhakat, S., Radadiya, A., Bhaskar, L. V. K. S., Shah, A., & Ragunath, P. K. (2016). Insights on the structural perturbations in human MTHFR Ala222Val mutant by protein modeling and molecular dynamics. Journal of Biomolecular Structure & Dynamics, 34(4), 892–905. https://doi.org/10.1080/07391102.2015.1057866
Adaikalakoteswari, A., Finer, S., Voyias, P. D., McCarthy, C. M., Vatish, M., Moore, J., Smart-Halajko, M., Bawazeer, N., Al-Daghri, N. M., McTernan, P. G., Kumar, S., Hitman, G. A., Saravanan, P., & Tripathi, G. (2015). Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clinical Epigenetics, 7(1), 14. https://doi.org/10.1186/s13148-015-0046-8
Bereket-Yücel, S. (2015). Creatine supplementation alters homocysteine level in resistance trained men. The Journal of Sports Medicine and Physical Fitness, 55(4), 313–319.
Bodenmann, S., Xu, S., Luhmann, U. F. O., Arand, M., Berger, W., Jung, H. H., & Landolt, H. P. (2009). Pharmacogenetics of modafinil after sleep loss: Catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clinical Pharmacology and Therapeutics, 85(3), 296–304. https://doi.org/10.1038/clpt.2008.222
Branched chain amino acids selectively promote cardiac growth at the end of the awake period. (2021). Journal of Molecular and Cellular Cardiology, 157, 31–44. https://doi.org/10.1016/j.yjmcc.2021.04.005
Bueno, O., Molloy, A. M., Fernandez-Ballart, J. D., García-Minguillán, C. J., Ceruelo, S., Ríos, L., Ueland, P. M., Meyer, K., & Murphy, M. M. (2016). Common polymorphisms that affect folate transport or metabolism modify the effect of the mthfr 677c > t polymorphism on folate status. The Journal of Nutrition, 146(1), 1–8. https://doi.org/10.3945/jn.115.223685
Chen, J., Lipska, B. K., Halim, N., Ma, Q. D., Matsumoto, M., Melhem, S., Kolachana, B. S., Hyde, T. M., Herman, M. M., Apud, J., Egan, M. F., Kleinman, J. E., & Weinberger, D. R. (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (Comt): Effects on mRNA, protein, and enzyme activity in postmortem human brain. American Journal of Human Genetics, 75(5), 807–821. https://doi.org/10.1086/425589
Choi, Y., Kim, J. O., Shim, S. H., Lee, Y., Kim, J. H., Jeon, Y. J., Ko, J. J., Lee, W. S., & Kim, N. K. (2016). Genetic variation of methylenetetrahydrofolate reductase (Mthfr) and thymidylate synthase (Ts) genes is associated with idiopathic recurrent implantation failure. PLoS ONE, 11(8), e0160884. https://doi.org/10.1371/journal.pone.0160884
Chung, J.-O., Lee, S.-B., Jeong, K.-H., Song, J.-H., Kim, S.-K., Joo, K.-M., Jeong, H.-W., Choi, J.-K., Kim, J.-K., Kim, W.-G., Shin, S.-S., & Shim, S.-M. (2018). Quercetin and fisetin enhanced the small intestine cellular uptake and plasma levels of epi-catechins in in vitro and in vivo models. Food & Function, 9(1), 234–242. https://doi.org/10.1039/c7fo01576c
Dietary quercetin exacerbates the development of estrogen-induced breast tumors in female ACI rats. (2010). Toxicology and Applied Pharmacology, 247(2), 83–90. https://doi.org/10.1016/j.taap.2010.06.011
El-Hadidy, M. A., Abdeen, H. M., Abd El-Aziz, S. M., & Al-Harrass, M. (2014). Mthfr gene polymorphism and age of onset of schizophrenia and bipolar disorder. BioMed Research International, 2014, 318483. https://doi.org/10.1155/2014/318483
Ganz, A. B., Shields, K., Fomin, V. G., Lopez, Y. S., Mohan, S., Lovesky, J., Chuang, J. C., Ganti, A., Carrier, B., Yan, J., Taeswuan, S., Cohen, V. V., Swersky, C. C., Stover, J. A., Vitiello, G. A., Malysheva, O. V., Mudrak, E., & Caudill, M. A. (2016). Genetic impairments in folate enzymes increase dependence on dietary choline for phosphatidylcholine production at the expense of betaine synthesis. The FASEB Journal, 30(10), 3321–3333. https://doi.org/10.1096/fj.201500138RR
García-Minguillán, C. J., Fernandez-Ballart, J. D., Ceruelo, S., Ríos, L., Bueno, O., Berrocal-Zaragoza, M. I., Molloy, A. M., Ueland, P. M., Meyer, K., & Murphy, M. M. (2014). Riboflavin status modifies the effects of methylenetetrahydrofolate reductase (Mthfr) and methionine synthase reductase (Mtrr) polymorphisms on homocysteine. Genes & Nutrition, 9(6), 435. https://doi.org/10.1007/s12263-014-0435-1
Hall, K. T., Buring, J. E., Mukamal, K. J., Vinayaga Moorthy, M., Wayne, P. M., Kaptchuk, T. J., Battinelli, E. M., Ridker, P. M., Sesso, H. D., Weinstein, S. J., Albanes, D., Cook, N. R., & Chasman, D. I. (2019). Comt and alpha-tocopherol effects in cancer prevention: Gene-supplement interactions in two randomized clinical trials. JNCI: Journal of the National Cancer Institute, 111(7), 684–694. https://doi.org/10.1093/jnci/djy204
Hall, K. T., Loscalzo, J., & Kaptchuk, T. J. (n.d.-a). Systems pharmacogenomics – gene, disease, drug and placebo interactions: A case study in COMT. Pharmacogenomics, 20(7), 529–551. https://doi.org/10.2217/pgs-2019-0001
Hall, K. T., Loscalzo, J., & Kaptchuk, T. J. (n.d.-b). Systems pharmacogenomics – gene, disease, drug and placebo interactions: A case study in COMT. Pharmacogenomics, 20(7), 529–551. https://doi.org/10.2217/pgs-2019-0001
Hall, K. T., Nelson, C. P., Davis, R. B., Buring, J. E., Kirsch, I., Mittleman, M. A., Loscalzo, J., Samani, N. J., Ridker, P. M., Kaptchuk, T. J., & Chasman, D. I. (2014). Polymorphisms in catechol-o-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(9), 2160–2167. https://doi.org/10.1161/ATVBAHA.114.303845
Husemoen, L. L. N., Skaaby, T., Jørgensen, T., Thuesen, B. H., Fenger, M., Grarup, N., Sandholt, C. H., Hansen, T., Pedersen, O., & Linneberg, A. (2014). MTHFR C677T genotype and cardiovascular risk in a general population without mandatory folic acid fortification. European Journal of Nutrition, 53(7), 1549–1559. https://doi.org/10.1007/s00394-014-0659-2
Hustad, S., Schneede, J., & Ueland, P. M. (2013). Riboflavin and methylenetetrahydrofolate reductase. Landes Bioscience. https://www.ncbi.nlm.nih.gov/books/NBK6145/
Jadavji, N. M., Emmerson, J. T., MacFarlane, A. J., Willmore, W. G., & Smith, P. D. (2017). B-vitamin and choline supplementation increases neuroplasticity and recovery after stroke. Neurobiology of Disease, 103, 89–100. https://doi.org/10.1016/j.nbd.2017.04.001
Kang, K. S., Yamabe, N., Wen, Y., Fukui, M., & Zhu, B. T. (2013). Beneficial effects of natural phenolics on levodopa methylation and oxidative neurodegeneration. Brain Research, 1497, 1–14. https://doi.org/10.1016/j.brainres.2012.11.043
Li, A., Shi, Y., Xu, L., Zhang, Y., Zhao, H., Li, Q., Zhao, X., Cao, X., Zheng, H., & He, Y. (2017). A possible synergistic effect of MTHFR C677T polymorphism on homocysteine level variations increased risk for ischemic stroke. Medicine, 96(51), e9300. https://doi.org/10.1097/MD.0000000000009300
Li, M.-N., Wang, H.-J., Zhang, N.-R., Xuan, L., Shi, X.-J., Zhou, T., Chen, B., Zhang, J., & Li, H. (2017). MTHFR C677T gene polymorphism and the severity of coronary lesions in acute coronary syndrome. Medicine, 96(49), e9044. https://doi.org/10.1097/MD.0000000000009044
Li, W.-X., Dai, S.-X., Zheng, J.-J., Liu, J.-Q., & Huang, J.-F. (2015). Homocysteine metabolism gene polymorphisms (Mthfr c677t, mthfr a1298c, mtr a2756g and mtrr a66g) jointly elevate the risk of folate deficiency. Nutrients, 7(8), 6670–6687. https://doi.org/10.3390/nu7085303
Li, Y., Qiu, S., Shi, J., Guo, Y., Li, Z., Cheng, Y., & Liu, Y. (2020). Association between MTHFR C677T/A1298C and susceptibility to autism spectrum disorders: A meta-analysis. BMC Pediatrics, 20, 449. https://doi.org/10.1186/s12887-020-02330-3
Liew, S.-C., & Gupta, E. D. (2015). Methylenetetrahydrofolate reductase (Mthfr) C677T polymorphism: Epidemiology, metabolism and the associated diseases. European Journal of Medical Genetics, 58(1), 1–10. https://doi.org/10.1016/j.ejmg.2014.10.004
Lisboa, J. V. de C., Ribeiro, M. R., Luna, R. C. P., Lima, R. P. A., do Nascimento, R. A. F., Monteiro, M. G. C. A., Lima, K. Q. de F., Fechine, C. P. N. dos S., de Oliveira, N. F. P., Persuhn, D. C., Veras, R. C., Gonçalves, M. da C. R., Ferreira, F. E. L. de L., Lima, R. T., da Silva, A. S., Diniz, A. da S., de Almeida, A. T. C., de Moraes, R. M., Verly Junior, E., & Costa, M. J. de C. (2020a). Food intervention with folate reduces tnf-α and interleukin levels in overweight and obese women with the mthfr c677t polymorphism: A randomized trial. Nutrients, 12(2), 361. https://doi.org/10.3390/nu12020361
Lisboa, J. V. de C., Ribeiro, M. R., Luna, R. C. P., Lima, R. P. A., do Nascimento, R. A. F., Monteiro, M. G. C. A., Lima, K. Q. de F., Fechine, C. P. N. dos S., de Oliveira, N. F. P., Persuhn, D. C., Veras, R. C., Gonçalves, M. da C. R., Ferreira, F. E. L. de L., Lima, R. T., da Silva, A. S., Diniz, A. da S., de Almeida, A. T. C., de Moraes, R. M., Verly Junior, E., & Costa, M. J. de C. (2020b). Food intervention with folate reduces tnf-α and interleukin levels in overweight and obese women with the mthfr c677t polymorphism: A randomized trial. Nutrients, 12(2), 361. https://doi.org/10.3390/nu12020361
Lok, A., Bockting, C. L. H., Koeter, M. W. J., Snieder, H., Assies, J., Mocking, R. J. T., Vinkers, C. H., Kahn, R. S., Boks, M. P., & Schene, A. H. (2013). Interaction between the MTHFR C677T polymorphism and traumatic childhood events predicts depression. Translational Psychiatry, 3(7), e288. https://doi.org/10.1038/tp.2013.60
Miller, R. J., Jackson, K. G., Dadd, T., Nicol, B., Dick, J. L., Mayes, A. E., Brown, A. L., & Minihane, A. M. (2012). A preliminary investigation of the impact of catechol-O-methyltransferase genotype on the absorption and metabolism of green tea catechins. European Journal of Nutrition, 51(1), 47–55. https://doi.org/10.1007/s00394-011-0189-0
Mthfr gene: Medlineplus genetics. (n.d.). Retrieved August 13, 2021, from https://medlineplus.gov/genetics/gene/mthfr/
Nowak, I., Bylińska, A., Wilczyńska, K., Wiśniewski, A., Malinowski, A., Wilczyński, J. R., Radwan, P., Radwan, M., Barcz, E., Płoski, R., Motak-Pochrzęst, H., Banasik, M., Sobczyński, M., & Kuśnierczyk, P. (2017). The methylenetetrahydrofolate reductase c.c.677 C>T and c.c.1298 A>C polymorphisms in reproductive failures: Experience from an RSA and RIF study on a Polish population. PLoS ONE, 12(10), e0186022. https://doi.org/10.1371/journal.pone.0186022
Rai, V. (2017). Association of C677T polymorphism (Rs1801133) in MTHFR gene with depression. Cellular and Molecular Biology (Noisy-Le-Grand, France), 63(6), 60–67. https://doi.org/10.14715/cmb/2017.63.6.13
Rai, V., Yadav, U., Kumar, P., Yadav, S. K., & Gupta, S. (2017). Methylenetetrahydrofolate reductase A1298C genetic variant & risk of schizophrenia: A meta-analysis. The Indian Journal of Medical Research, 145(4), 437–447. https://doi.org/10.4103/ijmr.IJMR_745_14
Sak, K. (2017a). The Val158Met polymorphism in COMT gene and cancer risk: Role of endogenous and exogenous catechols. Drug Metabolism Reviews, 49(1), 56–83. https://doi.org/10.1080/03602532.2016.1258075
Sak, K. (2017b). The Val158Met polymorphism in COMT gene and cancer risk: Role of endogenous and exogenous catechols. Drug Metabolism Reviews, 49(1), 56–83. https://doi.org/10.1080/03602532.2016.1258075
Sak, K. (2017c). The Val158Met polymorphism in COMT gene and cancer risk: Role of endogenous and exogenous catechols. Drug Metabolism Reviews, 49(1), 56–83. https://doi.org/10.1080/03602532.2016.1258075
Scoditti, E. (2020). Neuroinflammation and neurodegeneration: The promising protective role of the citrus flavanone hesperetin. Nutrients, 12(8). https://doi.org/10.3390/nu12082336
Stead, L. M., Au, K. P., Jacobs, R. L., Brosnan, M. E., & Brosnan, J. T. (2001). Methylation demand and homocysteine metabolism: Effects of dietary provision of creatine and guanidinoacetate. American Journal of Physiology. Endocrinology and Metabolism, 281(5), E1095-1100. https://doi.org/10.1152/ajpendo.2001.281.5.E1095
The extra virgin olive oil phenolic oleacein is a dual substrate-inhibitor of catechol-O-methyltransferase. (2019). Food and Chemical Toxicology, 128, 35–45. https://doi.org/10.1016/j.fct.2019.03.049
Troesch, B., Weber, P., & Mohajeri, M. H. (2016). Potential links between impaired one-carbon metabolism due to polymorphisms, inadequate b-vitamin status, and the development of alzheimer’s disease. Nutrients, 8(12), 803. https://doi.org/10.3390/nu8120803
Wan, L., Li, Y., Zhang, Z., Sun, Z., He, Y., & Li, R. (2018). Methylenetetrahydrofolate reductase and psychiatric diseases. Translational Psychiatry, 8, 242. https://doi.org/10.1038/s41398-018-0276-6
Wang, L.-J., Lee, S.-Y., Chen, S.-L., Chang, Y.-H., Chen, P. S., Huang, S.-Y., Tzeng, N.-S., Chen, K. C., Lee, I. H., Wang, T.-Y., Yang, Y. K., & Lu, R.-B. (2015). A potential interaction between COMT and MTHFR genetic variants in Han Chinese patients with bipolar II disorder. Scientific Reports, 5, 8813. https://doi.org/10.1038/srep08813
Wang, P., Heber, D., & Henning, S. M. (2012). Quercetin increased the antiproliferative activity of green tea polyphenol (−)-epigallocatechin gallate in prostate cancer cells. Nutrition and Cancer, 64(4), 580–587. https://doi.org/10.1080/01635581.2012.661514
Wu, X., Yang, K., Tang, X., Sa, Y., Zhou, R., Liu, J., Luo, Y., & Tang, W. (2015). Folate metabolism gene polymorphisms MTHFR C677T and A1298C and risk for preeclampsia: A meta-analysis. Journal of Assisted Reproduction and Genetics, 32(5), 797–805. https://doi.org/10.1007/s10815-014-0408-8
Xu, B., Kong, X., Xu, R., Song, Y., Liu, L., Zhou, Z., Gu, R., Shi, X., Zhao, M., Huang, X., He, M., Fu, J., Cai, Y., Li, P., Cheng, X., Wu, C., Chen, F., Zhang, Y., Tang, G., … Huo, Y. (2017). Homocysteine and all-cause mortality in hypertensive adults without pre-existing cardiovascular conditions. Medicine, 96(8), e5862. https://doi.org/10.1097/MD.0000000000005862
Yan, L., Zhao, L., Long, Y., Zou, P., Ji, G., Gu, A., & Zhao, P. (2012). Association of the maternal mthfr c677t polymorphism with susceptibility to neural tube defects in offsprings: Evidence from 25 case-control studies. PLoS ONE, 7(10), e41689. https://doi.org/10.1371/journal.pone.0041689
Yang, B., Fan, S., Zhi, X., Li, Y., Liu, Y., Wang, D., He, M., Hou, Y., Zheng, Q., & Sun, G. (2014). Associations of mthfr gene polymorphisms with hypertension and hypertension in pregnancy: A meta-analysis from 114 studies with 15411 cases and 21970 controls. PLoS ONE, 9(2), e87497. https://doi.org/10.1371/journal.pone.0087497