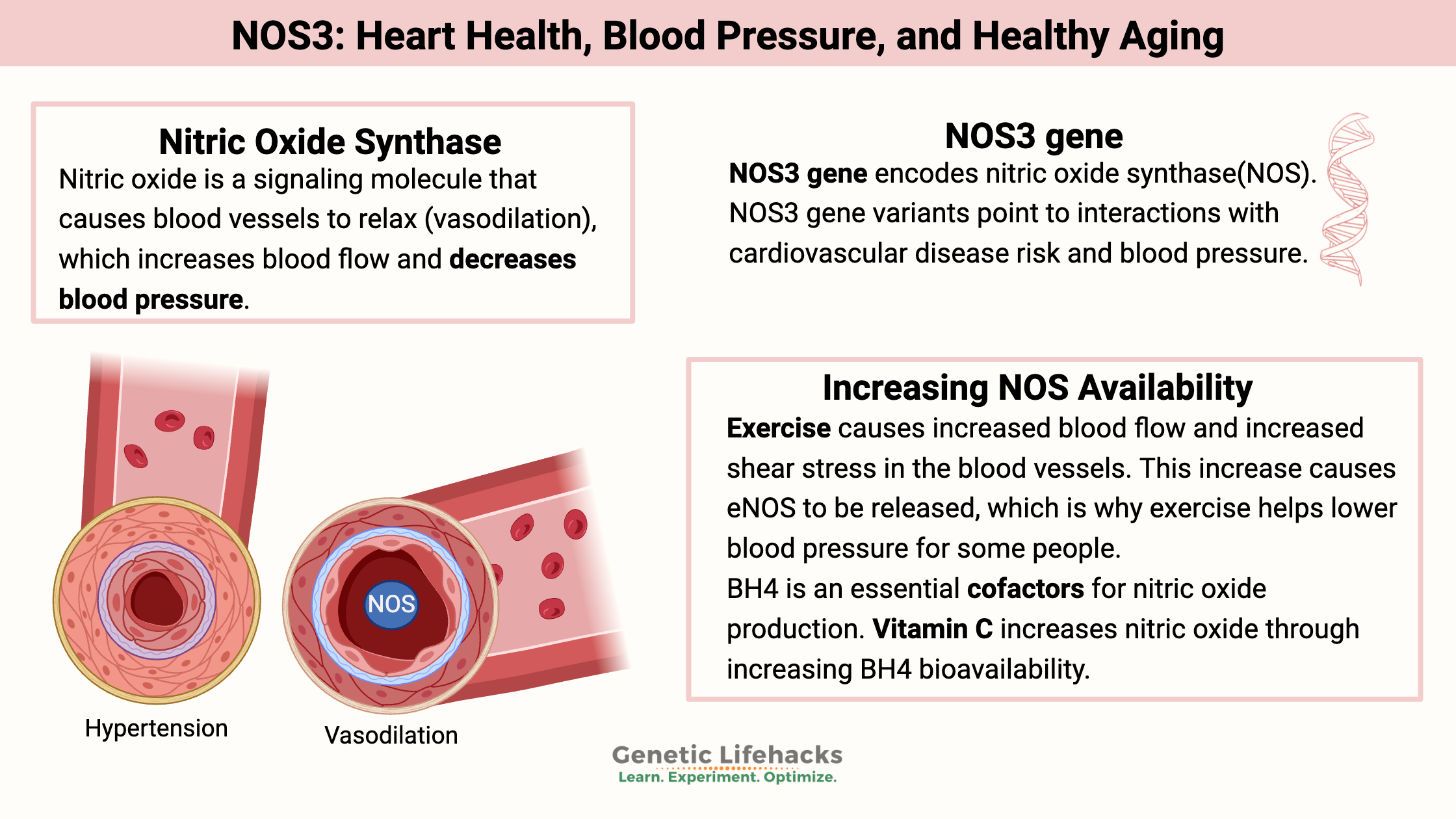
Key takeaways:
~ Nitric oxide is produced in the cells surrounding blood vessels to relax the blood vessels and lower blood pressure.
~ Decreased endothelial nitric oxide synthase (eNOS) can be a cause of heart disease, high blood pressure, and even affect brain health.
~ Genetic variants in the NOS3 gene are linked to lower eNOS and higher blood pressure.
The Endothelium and Nitric Oxide Synthase:
Let’s dig into the creation of nitric oxide, what it does in the body, and why it is so important for heart health and healthy aging.
Nitric oxide (NO) is a molecule that acts as a signaling molecule in the body. It is also a free radical, so the body tightly regulates it. The body creates nitric oxide in specific reactions, but it only hangs around for less than 5 seconds.[ref]
Quick aside: Nitric oxide is not the same thing as nitrous oxide (laughing gas).
What does nitric oxide do in the body?
Nitric oxide’s composition consists of a nitrogen atom bound to an oxygen atom. This pairing leaves one electron free, thus making it a free radical.
Nitric oxide in the environment reacts to form acid rain, participates in ozone layer depletion, and it can kill you if you breathe it at high concentrations. Same molecule, but different roles when created inside the body.
Nitric oxide is a tiny molecule; it can diffuse across cell membranes and move without needing to be transported. This tiny gaseous NO molecule acts as a signaling molecule in pretty much every living organism (bacteria, plants, fungi, animals).
Vasodilation:
One important role that nitric oxide plays in the body is to act as a signaling molecule from the endothelium to the surrounding layers of the blood vessels, causing the blood vessel to relax. This relaxation (vasodilation) increases blood flow. Vasodilation usually causes a decrease in blood pressure.
Producing nitric oxide in the endothelium:
We need nitric oxide at the right amount in the endothelial cells that make up blood vessels.
The enzyme responsible for producing nitric oxide in the endothelium is known as eNOS (endothelium nitric oxide synthase), and it is coded for by the NOS3 gene.
Acetylcholine signals the creation of eNOS, and then the nitric oxide synthase causes the creation of nitric oxide from the amino acid arginine.
The NO produced in the endothelium regulates the constriction of the blood vessels, the stickiness of platelets, and leukocyte adhesion. It can diffuse from the endothelial cells into neighboring smooth muscle cells, causing the muscle to relax.
Let’s pause for a moment here for a quick explanation of the endothelium and then get into the chemistry of creating NO.
Background: What is the endothelium?
The single layer of endothelial cells forming the lining of blood vessels and lymph vessels is called the endothelium. This thin cell layer is essential to the health of your blood vessels. Endothelial cells can divide, and if there is a tear in a blood vessel, neighboring endothelial cells can proliferate and repair the tear. The endothelium also assists in controlling the rate of blood flow.[ref]
The endothelium acts as a barrier between everything in the bloodstream and the rest of the tissues of the body. But it is way more than just a passive lining.
Endothelial cells are selectively permeable – allowing in certain chemicals and white blood cells, and giving off signaling molecules.
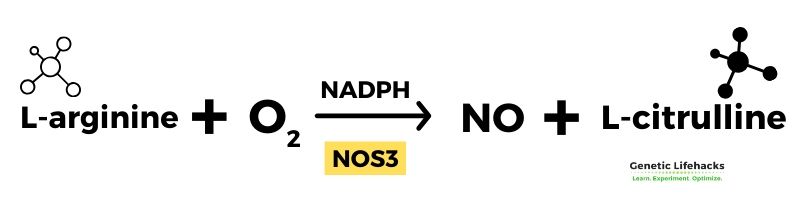
Creation of nitric oxide: arginine, oxygen, citrulline
Arginine, an amino acid, converts into nitric oxide with the addition of oxygen. The product of the reaction is l-citrulline and NO.
Two essential proteins needed in the reaction are tetrahydrobiopterin (BH4) and nitric oxide synthase (NOS3, eNOS). Nitric oxide synthase (eNOS) is the enzyme that catalyzes the reaction.
Nitric oxide synthase turns up in other areas of the body, so in the endothelium, nitric oxide synthase (NOS) is referred to as eNOS to differentiate it from NOS elsewhere.
eNOS (NOS3) consists of two identical proteins joined together with BH4, a cofactor essential to this process. Without BH4 creating the bond, the reaction using just one nitric oxide synthase molecule will actually create a superoxide anion, which is a deleterious free radical. Bad stuff.[ref][ref]
When NOS joins together (with BH4 to help), it is called ‘coupled’. When not joined together, it is called uncoupled eNOS.
Excess oxidative stress is a driver of cardiovascular disease. One problem with oxidative stress is that it diminishes the levels of BH4. Without BH4 coupling the NOS3 molecules together, the reaction to form nitric oxide from l-arginine cannot happen. Instead, this results in the production of superoxide, a source of oxidative stress in the cell. Thus, excess oxidative stress decreases NOS3, driving the production of more oxidative stress.[ref]
What causes nitric oxide to be released?
The endothelium controls nitric oxide formation in multiple ways.
- The blood flowing through your veins causes a shear force on the endothelium, and this mechanical force acts to regulate NO release. It goes on all day long, every day. Plus, this blood flow regulation keeps platelets from activating and sticking together.[ref]
- You can have a decrease in eNOS due to a lack of oxygen (hypoxia) or due to insufficient BH4. A lack of eNOS causes blood pressure to rise. Alternatively, too much eNOS is also detrimental, so balance is needed.[ref]
- Hyperglycemia, or high blood sugar, can also inhibit eNOS. It may play a significant role in cardiovascular problems in people with diabetes.[ref]
Histamine (H1) receptors on the endothelial cell cause nitric oxide to be released in response to histamine. It is how histamine increases vascular permeability.[ref][ref]
Nitric oxide, endothelial function, and aging:
Cardiovascular disease (CVD) is the #1 cause of death in most countries, and about 70% of people over age 60 meet the criteria for CVD.
Researchers discovered decades ago that nitric oxide production in the endothelial cells lining the arteries is key to the health of the cardiovascular system.
As we age, there is an increase in oxidative stress in the body and a decrease in nitric oxide bioavailability. This reduction in nitric oxide directly drives the stiffening of the arteries seen in CVD.[ref]
Cardiovascular disease can arguably be considered a problem of imbalanced reactive oxygen species – e.g., oxidative stress. A simplified explanation is that this increase in oxidative stress decreases eNOS, thus increasing blood pressure.[ref]
What causes the increase in ROS over what the cell needs as we age? Things like chronic inflammation, mold toxins, gum disease, and heavy metals.
Exercise, heart health, and NOS3
Good blood flow and oxygen transport are essential for athletic performance, and nitric oxide is one variable athletes target to increase their endothelial function.
Exercise causes increased blood flow and increased shear stress in the blood vessels. This increase causes eNOS to be released, which is why exercise helps lower blood pressure for some people.[ref]
NOS3 Genotype Report
Lifehacks:
Nitric oxide is needed at just the right levels, specific to the tissue in question.
Increasing endothelial nitric oxide synthase may help with hypertension for some people. It may especially be true in conjunction with the above NOS3 genetic variants.[ref]
Natural supplements for Increasing BH4 or NOS availability:
BH4 (tetrahydrobiopterin) is an essential cofactor for nitric oxide production. Your body naturally produces BH4, and under normal conditions, it gets recycled and reused in the cell. But when oxidative stress is high (excess reactive oxygen species), it can be used up more quickly.[ref]
Related Articles and Topics:
References:
Afef, Letaief, et al. “Endothelial Nitric Oxide Gene Polymorphisms and Their Association with Coronary Artery Disease in Tunisian Population.” Anatolian Journal of Cardiology, vol. 17, no. 1, Jan. 2017, pp. 31–36. PubMed, https://doi.org/10.14744/AnatolJCardiol.2016.6946.
Alberts, Bruce, et al. “Blood Vessels and Endothelial Cells.” Molecular Biology of the Cell. 4th Edition, 2002. www.ncbi.nlm.nih.gov, https://www.ncbi.nlm.nih.gov/books/NBK26848/.
Aminuddin, Farzian, et al. “Nitric Oxide Synthase Polymorphisms, Gene Expression and Lung Function in Chronic Obstructive Pulmonary Disease.” BMC Pulmonary Medicine, vol. 13, Nov. 2013, p. 64. PubMed, https://doi.org/10.1186/1471-2466-13-64.
Conen, David, et al. “Association of Renin-Angiotensin and Endothelial Nitric Oxide Synthase Gene Polymorphisms with Blood Pressure Progression and Incident Hypertension: Prospective Cohort Study.” Journal of Hypertension, vol. 26, no. 9, Sept. 2008, pp. 1780–86. PubMed, https://doi.org/10.1097/HJH.0b013e3283077eef.
Di Francescomarino, Samanta, et al. “The Effect of Physical Exercise on Endothelial Function.” Sports Medicine (Auckland, N.Z.), vol. 39, no. 10, 2009, pp. 797–812. PubMed, https://doi.org/10.2165/11317750-000000000-00000.
Du, X. L., et al. “Hyperglycemia Inhibits Endothelial Nitric Oxide Synthase Activity by Posttranslational Modification at the Akt Site.” The Journal of Clinical Investigation, vol. 108, no. 9, Nov. 2001, pp. 1341–48. PubMed, https://doi.org/10.1172/JCI11235.
Ferguson, Jane F., et al. “NOS3 Gene Polymorphisms Are Associated with Risk Markers of Cardiovascular Disease, and Interact with Omega-3 Polyunsaturated Fatty Acids.” Atherosclerosis, vol. 211, no. 2, Aug. 2010, pp. 539–44. PubMed, https://doi.org/10.1016/j.atherosclerosis.2010.03.027.
Förstermann, Ulrich, and Thomas Münzel. “Endothelial Nitric Oxide Synthase in Vascular Disease: From Marvel to Menace.” Circulation, vol. 113, no. 13, Apr. 2006, pp. 1708–14. PubMed, https://doi.org/10.1161/CIRCULATIONAHA.105.602532.
Hennekens, Charles H., et al. “A Randomized Trial of Aspirin at Clinically Relevant Doses and Nitric Oxide Formation in Humans.” Journal of Cardiovascular Pharmacology and Therapeutics, vol. 15, no. 4, Dec. 2010, pp. 344–48. DOI.org (Crossref), https://doi.org/10.1177/1074248410375091.
Incalza, Maria Angela, et al. “Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases.” Vascular Pharmacology, vol. 100, Jan. 2018, pp. 1–19. PubMed, https://doi.org/10.1016/j.vph.2017.05.005.
Kamal Patel, M. P. H. Arginine Research Analysis. Sept. 2022. examine.com, https://examine.com/supplements/arginine/.
Li, Huige, et al. “Histamine Upregulates Gene Expression of Endothelial Nitric Oxide Synthase in Human Vascular Endothelial Cells.” Circulation, vol. 107, no. 18, May 2003, pp. 2348–54. PubMed, https://doi.org/10.1161/01.CIR.0000066697.19571.AF.
Luo, Suxin, et al. “Molecular Mechanisms of Endothelial NO Synthase Uncoupling.” Current Pharmaceutical Design, vol. 20, no. 22, 2014, pp. 3548–53. PubMed, https://doi.org/10.2174/13816128113196660746.
Melik, Ziva, et al. “L-Arginine as Dietary Supplement for Improving Microvascular Function.” Clinical Hemorheology and Microcirculation, vol. 65, no. 3, 2017, pp. 205–17. PubMed, https://doi.org/10.3233/CH-16159.
Mortensen, Alan, and Jens Lykkesfeldt. “Does Vitamin C Enhance Nitric Oxide Bioavailability in a Tetrahydrobiopterin-Dependent Manner? In Vitro, in Vivo and Clinical Studies.” Nitric Oxide: Biology and Chemistry, vol. 36, Jan. 2014, pp. 51–57. PubMed, https://doi.org/10.1016/j.niox.2013.12.001.
Santos-Parker, Jessica R., et al. “Curcumin Supplementation Improves Vascular Endothelial Function in Healthy Middle-Aged and Older Adults by Increasing Nitric Oxide Bioavailability and Reducing Oxidative Stress.” Aging, vol. 9, no. 1, Jan. 2017, pp. 187–208. PubMed, https://doi.org/10.18632/aging.101149.
Seelenfreund, D., et al. “Association of the Intronic Polymorphism Rs891512 (G24943A) of the Endothelial Nitric Oxide Synthase Gene with Hypertension in Chilean Type 2 Diabetes Patients.” Diabetes Research and Clinical Practice, vol. 96, no. 2, May 2012, pp. e47-49. PubMed, https://doi.org/10.1016/j.diabres.2012.01.029.
Singh, Puneetpal, et al. “Investigation of ENOS Gene Polymorphism Exposes a Genetic Association between Endothelial Dysfunction and Osteoporosis in Postmenopausal Women.” Menopause (New York, N.Y.), vol. 27, no. 6, June 2020, pp. 714–21. PubMed, https://doi.org/10.1097/GME.0000000000001514.
Stanhewicz, Anna E., et al. “Folic Acid Supplementation Improves Microvascular Function in Older Adults through Nitric Oxide-Dependent Mechanisms.” Clinical Science (London, England: 1979), vol. 129, no. 2, July 2015, pp. 159–67. PubMed, https://doi.org/10.1042/CS20140821.
Vimaleswaran, Karani S., et al. “Habitual Energy Expenditure Modifies the Association between NOS3 Gene Polymorphisms and Blood Pressure.” American Journal of Hypertension, vol. 21, no. 3, Mar. 2008, pp. 297–302. PubMed, https://doi.org/10.1038/ajh.2007.69.
Wang, Linhong, et al. “Association Study of NOS3 Gene Polymorphisms and Hypertension in the Han Chinese Population.” Nitric Oxide: Biology and Chemistry, vol. 51, Dec. 2015, pp. 1–6. PubMed, https://doi.org/10.1016/j.niox.2015.09.004.
Wang, Qiuling, et al. “ENOS Rs2070744 Polymorphism Might Influence Predisposition to Hemorrhagic Cerebral Vascular Diseases in East Asians: A Meta-Analysis.” Brain and Behavior, vol. 10, no. 5, May 2020, p. e01538. PubMed, https://doi.org/10.1002/brb3.1538.
Wilmink, H. W., et al. “Influence of Folic Acid on Postprandial Endothelial Dysfunction.” Arteriosclerosis, Thrombosis, and Vascular Biology, vol. 20, no. 1, Jan. 2000, pp. 185–88. PubMed, https://doi.org/10.1161/01.atv.20.1.185.
Yao, Yingshui, et al. “Evaluation of Genetic Effect of NOS3 and G×E Interaction on the Variability of Serum Bilirubin in a Han Chinese Population.” Nitric Oxide: Biology and Chemistry, vol. 70, Nov. 2017, pp. 25–30. PubMed, https://doi.org/10.1016/j.niox.2017.08.002.
Zhang, Yi-Qing, et al. “Role of Endothelial Nitric Oxide Synthase Polymorphisms in Atrial Fibrillation: A PRISMA-Compliant Meta-Analysis.” Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, vol. 25, Apr. 2019, pp. 2687–94. PubMed, https://doi.org/10.12659/MSM.913528.
https://academic.oup.com/nutritionreviews/article/75/1/61/2684504. Accessed 19 Oct. 2022.