Key takeaways:
~Nutrigenomics – nutrition combined with genetics – is important in pregnancy.
~ Genetic variants affect how mothers process essential nutrients like choline, folate, and DHA/EPA, and this can potentially affect the baby’s development.
~ Environmental factors, including mercury exposure and iron levels, can also interact with genetics.
Nutrigenomics and Pregnancy:
A mother’s diet during pregnancy is fundamental to the development of her baby. During pregnancy, there is an increased need for vitamins, choline, folate, DHA, and overall nutrients.[ref]
This article covers some of the known genetic interactions between the mother’s genetic variants and her diet, and shows the effects on the child. You can use this information to understand which nutrients are most important for you to ensure that you have adequate coverage.
However, it is also important to know that excessive supplementation, even of water-soluble vitamins, could have negative effects on the developing fetus.
Pregnancy is not the time to go overboard with supplements or major dietary changes. Talk with your doctor for any medical advice, and please take into consideration the RDA for pregnant women when considering supplements in addition to your dietary intake.
Below you will find seven sections that explain specific nutrient and toxin interactions with genetic variants. I’ll close with some interesting studies on environmental factors in pregnancy.
Choline: Brain Development and More
Choline is an essential nutrient for the developing fetus, used for the development of cell membranes and neurons, and as an alternative source of methyl groups to folate. Interestingly, the placenta stores a large amount of choline so that it can be easily delivered to the growing baby.[ref]
Synthesizing choline vs. dietary choline:
The liver can synthesize choline, and we get choline from our diets (eggs, meat, seafood, spinach, and beets are good sources). During pregnancy, the body’s production of choline is increased and transported to the developing baby, often leaving the mother deficient. Thus, during pregnancy, more choline is needed from dietary sources to supplement what the body is able to synthesize. Despite the increased production of choline, pregnant women end up low in choline because it is preferentially transported to the developing baby. Breast milk is also high in choline, so breastfeeding the baby also depletes the mother of choline.[ref][ref]
A US national survey showed that over 90% of the adult population doesn’t meet the Adequate Intake (AI) recommendations for choline. The AI is 550 mg/day for men and 425 mg/day for women who aren’t pregnant. During pregnancy and lactation, the AI increases to 550 mg of choline per day.[ref].
Choline in the methylation cycle:
Doctors and researchers first realized that adequate folate reduced the incidence of neural tube defects such as spina bifida. Folic acid fortification of cereals, bread, pasta, white rice, and tortillas was implemented in many countries. This fortification of processed foods reduced the rate of neural tube defects by increasing the folic acid intake of women before pregnancy (along with everyone else in the country). The nationwide fortification of grains with folic acid was estimated to prevent 1,000 cases of neural tube defects in the years after it was implemented, but a CDC study showed that there were still about 3,000 babies born with neural tube defects each year, even with the mandated fortification.[ref]
Choline in brain development:
Choline is an essential component in brain development, and the maternal choline intake affects the infant’s brain later in life. A small randomized controlled trial looked at whether increasing choline intake during pregnancy could improve cognitive processing time later for the baby. The study participants consumed either 480 mg choline/day or 930 mg choline/day during their third trimester. The researchers followed up with the infants over the next year after birth, testing their visuospatial memory and information processing speed. The group whose mothers had consumed 930 mg/day of choline had faster scores on both measures.[ref] However, not all studies agree. Studies in older children don’t show a statistical advantage in children’s IQ scores based on maternal choline intake.[ref]
Genetic connections with choline:
PEMT gene: codes for the enzyme phosphatidylethanolamine N-methyltransferase, which is key in the body’s ability to create choline. Genetic variants that decrease the function of the enzyme cause a greater reliance on choline from dietary sources.
Check your genetic data for rs7946 V175M (23andMe v4, v5; AncestryDNA):
- C/C: typical PEMT activity (most common genotype worldwide)
- C/T: typical risk
- T/T: decreased PEMT enzyme activity[ref]; increased relative risk of preterm birth with low choline intake[ref]
Members: Your genotype for rs7946 is —.
Check your genetic data for rs12325817 (AncestryDNA – older files):
- C/C: typical
- C/G: increased risk of organ dysfunction with low choline diet[ref]
- G/G: increased risk of organ dysfunction with low choline diet, lower betaine levels in pregnant women with inadequate choline intake[ref]
Members: Your genotype for rs12325817 is —.
BHMT gene: encodes betaine homocysteine S-methyltransferase, which is an enzyme that helps to convert homocysteine into methionine using betaine.
Check your genetic data for rs3733890 R239Q G716A (23andMe v4, v5; AncestryDNA):
- A/A: reduced BHMT[ref][ref] reduced conversion of choline to betaine[ref] increased risk of early-onset heart disease with poor diet[ref]
- A/G: reduced BHMT, reduced conversion of choline to betaine, increased risk of early-onset heart disease with poor diet
- G/G: typical
Members: Your genotype for rs3733890 is —.
Check your genetic data for rs651852 (23andMe v4 only):
- C/C: increased risk of cleft lip, an indicator of low methyl groups[ref]
- C/T: typical
- T/T: typical
Members: Your genotype for rs651852 is —.
Foods high in choline:
Good sources of dietary choline include eggs, beef liver, beef steaks, soybeans, chicken, and fish. Plant-based sources of choline include potatoes, wheat germ, and kidney beans.[ref]
Types of choline:
There are several types of choline found in food and in supplements:
- Betaine (also called trimethylglycine or TMG) can easily be used in cells as a methyl donor. It is found in foods like spinach, beets, and seafood, or your cells can convert other forms of choline into betaine.
- CDP choline (Citicoline) – used by the body as a precursor for phosphatidylcholine
- Alpha-GPC choline – semisynthetic form derived from lecithin that is more bioavailable than citicoline
- Phosphatidylcholine – this is the form that makes up cell membranes throughout the body and is easily absorbed as a supplement. Foods containing phosphatidylcholine include eggs, beef, chicken, fish, and liver.
- Choline bitartrate – inexpensive form found in supplements but not as well absorbed
Related article: Which type of choline is best
Folate: Preventing NTDs and More
Folate is essential for fetal and placental growth. It plays an important role in DNA synthesis, brain and nervous system development, and proper methylation of genes during development. The WHO recommends 400 mcg/day of folic acid, and the U.S. RDA is 600 mcg/day of folate equivalents for pregnancy.[ref][ref]
Explanation of recommended amounts:
Folic acid and methylfolate are more readily absorbed than folate from foods. The U.S. RDAs are now based on the equivalent amount of folate from food.
Folic acid and methylfolate are equal to about 1.6 folate equivalents, with 240 mcg of folic acid equal to 400 mcg of folate equivalents.
Neural tube defects (NTDs):
Folic acid fortification of grains began in the mid-1990s based on studies showing that adequate folic acid intake during pregnancy is necessary to prevent neural tube defects such as spina bifida. The neural tube closes around day 28 of pregnancy, so adequate folate (or choline) is needed very early, often before women know they are pregnant.[ref]
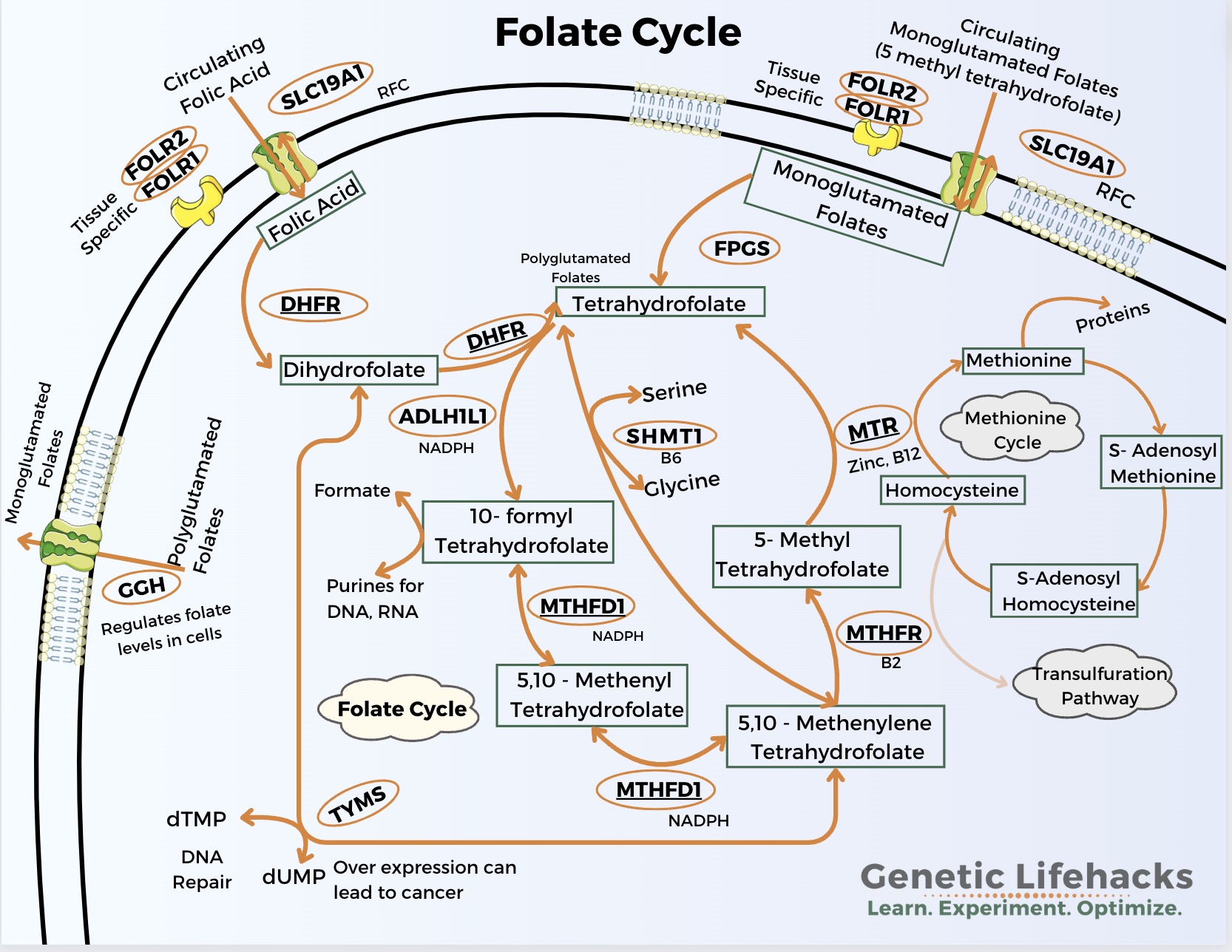
Methylation and folate cycle:
Several studies have looked at the folate-related genetic variants (MTHFR, MTHFD1, MTR, MTRR) and the effect of low maternal folate on the risk of low birth weight, preterm birth, or small for gestational age. Some studies find no effect of maternal genetic variants in conjunction with folate intake, but other studies do show that genetic variants interact with folate intake and the size of the baby at birth.[ref][ref]
Congenital heart disease:
Maternal folate intake is also protective against congenital heart disease (CHD) in their children. One study referenced below also looked at the baby’s genotype for a variant in the MTRR gene and showed that it interacts with maternal folate intake in the risk of CHD.[ref]
Genetic connections with folate:
MTHFR gene: encodes a key enzyme in the folate cycle
Check your genetic data for rs1801133 (23andMe v4, v5; AncestryDNA):
- G/G: typical
- A/G: one copy of MTHFR C677T allele, enzyme function decreased by 40%; increased risk of neural tube defects with low folate intake[ref]; increased relative risk of preeclampsia[ref][ref]
- A/A: two copies of MTHFR C677T, enzyme function decreased by 70 – 80%[ref]; increased relative risk of preterm birth or low birth weight[ref]; increased risk of neural tube defects with low folate intake[ref]; increased relative risk of preeclampsia[ref]
Members: Your genotype for rs1801133 is —.
Check your genetic data for rs1801131 (23andMe v4, v5; AncestryDNA):
- T/T: typical *
- G/T: one copy of MTHFR A1298C (heterozygous), slightly decreased enzyme function
- G/G: two copies of MTHFR A1298C (homozygous), decreased enzyme by about 20%; increased relative risk of small for gestational age baby without adequate maternal folate in pregnancy[ref]
Members: Your genotype for rs1801131 is —.
Check your genetic data for rs2274976 G1793A or R594Q (23andMe v4, v5; AncestryDNA):
- T/T: decreased MTHFR efficiency; 3-fold increased risk of cleft lip[ref], increased risk of neural tube defect[ref], higher homocysteine, and risk of folate deficiency[ref]
- C/T: increased risk of cleft lip and neural tube defects
- C/C: typical
Members: Your genotype for rs2274976 is —.
Check your genetic data for rs17367504 (23andMe v4, v5; AncestryDNA):
- G/G: protective against hypertension, reduced risk of preeclampsia[ref][ref]
- A/G: protective against hypertension, preeclampsia
- A/A: typical
Members: Your genotype for rs17367504 is —.
MTHFD1 gene: The MTHFD1 gene codes for the enzyme called methylenetetrahydrofolate dehydrogenase, cyclohydrolase, and formyltetrahydrofolate synthetase 1, which is part of the folate pathway.
Check your genetic data for rs2236225 (G1958A): (23andMe v4, v5; AncestryDNA):
- A/A: decreased MTHFD1 enzyme stability[ref], more of a reliance on choline as a methyl donor[ref][ref], increased risk of congenital heart disease in offspring[ref]
- A/G: decreased MTHFD1 enzyme stability[ref], more of a reliance on choline as a methyl donor
- G/G: typical
Members: Your genotype for rs2236225 is —.
Can you get too much folate?
In pregnancy, the RDA is 400 μg/day of folic acid (equivalent to 600 mcg/day of folate from food).[ref] Going beyond this with a lot of excess folate may not be benign. The tolerable upper limit for folate in pregnancy set by the FDA is 1,000 mg/day of folate equivalents, which would be 600 mcg of folic acid.
A meta-analysis of 18 studies on folic acid in pregnancy notes that supplementation is associated with an increased relative risk of asthma in children. From the study: “The risk of asthma in children significantly increased when maternal folic acid intake reached 581 μg/day.”[ref]
High doses of folic acid increased the relative risk of childhood cancer 3-fold in children whose mothers had epilepsy and took 1 mg of folic acid. However, there was no increased risk of cancer in children whose mothers had epilepsy but didn’t take folic acid. In the group of mothers without epilepsy who took 1 mg of folic acid, there was a smaller (~10%) increased relative risk of childhood cancer.[ref]
Keep in mind that these two examples involve relative risk — the absolute risk of childhood cancer is still small. Getting too much folic acid periodically is not likely to be harmful.
My point here is that excessive folic acid or methylfolate supplementation may not be necessary and may carry small risks. Again, talk to your doctor if you have any questions.
Sources of folate:
Naturally folate-rich foods include legumes, green vegetables, and beef liver. Supplemental folate in prenatal vitamins is usually available as either methylfolate or folic acid. Methylfolate is the active form that is more readily used in cells, while folic acid is a form that must go through several steps to be converted to methylfolate in cells.
Cronometer.com is a free web app where you can track what you eat for a few days to see how much folate you are getting. If you are eating fortified grains, it can add up. For example, one serving (1 c) of Cheerios has 80 mcg of folate, and two slices of white bread have about 100 mcg of folate. One Chipotle burrito has 166 mcg of folate. [ref]
Related articles: MTHFR and Folic Acid conversion by DHFR
DHA and EPA: Eye and Brain Development
DHA and EPA are omega-3 fatty acids found abundantly in fish and seafood as well as in pasture-raised eggs and grass-fed beef. These omega-3 fatty acids play an essential role in the formation of the cell membranes in the nerve cells of the developing brain.
There’s a higher need for DHA throughout pregnancy, but the baby’s DHA needs peak in the third trimester of development. One study showed that maternal DHA supplementation of 600 mg/day from week 20 onward resulted in better outcomes and increased birth weight. Studies also show that maternal DHA supplementation directly affects the baby’s DHA status, improving brain and eye health.[ref]
Stack choline with DHA:
Choline supplementation along with DHA has synergistic benefits. A study involving pregnant women showed that supplemental choline plus DHA improved the DHA status more because of the beneficial effect of choline on the methylation cycle, improving DHA export from the liver. [ref][ref]
DHA/EPA from plants or from fish?
The FADS1 and FADS2 genes encode the enzymes utilized by cells for converting the plant forms of omega-3 (such as from flax seed or chia seeds) into the longer chain forms, including DHA and EPA.
Common polymorphisms in the FADS1 and FADS2 genes are associated with lower levels of DHA and EPA in people who don’t consume much fish or seafood in their diets. These genetic variants are also associated with lower DHA and EPA levels during pregnancy. DHA and EPA are also essential for infant brain development during lactation, so continuing with omega-3 intake during breastfeeding is important.
Genetic Connections with DHA and EPA
FADS1 gene: encodes delta-5 desaturase or Fatty acid desaturase 1, which converts plant-based omega-3s, such as from flax seed, into DHA and EPA
There are multiple FADS1 variants that are inherited together such that if you inherit one, you should inherit all the variants.
Check your genetic data for rs174546 (23andMe v4, v5; AncestryDNA):
- T/T: low FADS1 enzyme activity; likely to have low DHA and EPA levels during pregnancy if not supplementing[ref]
- C/T: lower FADS1 enzyme activity,
- likely to have low DHA and EPA levels during pregnancy if not supplementing[ref]
- C/C: typical FADS1 activity
Members: Your genotype for rs174546 is —.
FADS2 gene: encodes fatty acid desaturase 2, which converts plant-based omega-3s, such as from flax seed, into DHA and EPA
Check your genetic data for rs1535 (23andMe v4, v5; AncestryDNA):
- G/G: low FADS2 enzyme activity, decreased ALA to EPA conversion[ref], likely to have low DHA and EPA levels during pregnancy if not supplementing[ref]
- A/G: somewhat decreased FADS2 enzyme activity
- A/A: typical FADS2
Members: Your genotype for rs1535 is —.
Supplemental DHA and EPA:
Flaxseed oil or chia seeds are partially converted to DHA and EPA for people with typical FADS1 and FADS2 function. If you have lower FADS1/2 activity, you may not be able to meet your DHA needs in pregnancy without either eating fish or supplementing directly.
Supplementing with DHA and EPA is a good way to boost your intake, especially if you are worried about mercury in fish. ConsumerLab.com does independent testing of supplements to see if they contain what is on the label and whether they are free of heavy metals. For fish and krill oil, they also tested for freshness (lack of oxidation). The ConsumerLab top picks were Carlson’s Maximum Omega 2000, Kirkland (Costco) Fish oil, Carlson’s The Very Finest Fish Oil, Nordic Natural Ultimate Omega, Barlean’s Omega Pals, and Deva Vegan Omega-3. There are many other brands that also passed their freshness and heavy metal tests. However, Wiley’s Finest Wild Alaskan Prenatal and Jamieson Omega-3 Complete did not pass the freshness (oxidation) test.
Krill oil and algae oil are also good sources of DHA and EPA. If you are looking for a vegetarian option, algae oils are a great source of DHA and EPA, but they are a little more expensive.
Related article: FADS1 and FADS2 in the conversion of omega-3 and omega-6
DHA in development affects Reelin:
Low DHA (omega-3) levels are a problem during fetal brain development. Interestingly, low DHA interacts with reelin to cause a temporary partial deficiency.[ref] Reelin deficiency is one reason that getting enough DHA is important in pregnancy.
Related article: Reelin and brain development
Mercury detoxification:
Mercury is a major concern for developing brains. A study of women who ate a lot of fish and had high levels of mercury in their hair showed that their offspring were more likely to have slightly impaired mental development.
Glutathione is an antioxidant that plays an important role in detoxifying mercury. A variant in the GSTP1 gene, which is responsible for catalyzing detoxification reactions with glutathione, is associated with negative outcomes in the baby from high maternal mercury intake. [ref]
Fish intake and mercury: A conundrum
Fish consumption is important for DHA and EPA in women who are not supplementing with an omega-3 supplement. The obvious problem here is that mercury in fish is harmful to the baby. General guidelines for pregnancy are to eat 2-3 servings of fish or seafood low in mercury each week.[ref]
If you are eating locally caught fish, be sure to check for specific guidelines for water bodies in your area (here’s an example for California). The FDA has general guidelines on fish consumption during pregnancy and childhood. They list tuna, king mackerel, orange roughy, marlin, shark, and swordfish as fish to avoid due to high levels of mercury.
Genetic Connections with Mercury Detoxification
GSTP1 gene: encodes glutathione-s-transferase, which is important in the phase II detoxification and excretion of mercury
Check your genetic data for rs1695 (23andMe v4, v5; AncestryDNA):
- A/A: typical; possibly higher IL-6 in men who take vitamin E[ref]
- A/G: typical risk of breast cancer
- G/G: reduced function, [ref][ref]; lower mental development scores in children when maternal diet has a high intake of fish containing mercury[ref]
Members: Your genotype for rs1695 is —.
Related article: Mercury detoxification
Iron: Essential in the right amount
Iron absorption and iron levels are carefully balanced by the body — having sufficient iron is essential for oxygen transport, but too much iron increases oxidative stress and can eventually cause organ damage.
Iron deficiency during pregnancy is associated with an increased relative risk of intrauterine growth retardation, low birth weight, or premature labor.[ref]
Manganese and Iron:
Genetic variants in the HFE gene can cause higher iron levels, which may be beneficial during pregnancy but can be a problem later in life with iron accumulation causing oxidative stress.
Iron and manganese use the same transporters for absorption. A Harvard study showed that variants in the HFE gene that can cause hemochromatosis are associated with high iron absorption and lower manganese levels in pregnant women.[ref]
Genetic connections with iron:
TMPRSS6 gene: encodes a protease that regulates hepcidin and iron levels
Check your genetic data for rs855791 (23andMe v4, v5):
- A/A: 4-fold increased relative risk of iron-deficient anemia in pregnancy with low iron intake[ref]
- A/G: typical risk
- G/G: typical
HFE gene: codes for a protein that controls how iron is absorbed in the intestines.[ref] Mutations here can cause hemochromatosis or iron overload. This interaction with increased iron can cause lower manganese levels. If you have the one copy of the C282Y variant or two copies of the H63D variant, please read the full Hemochromatosis article.
Check your genetic data for rs1800562 C282Y (23andMe v4, v5; AncestryDNA):
- A/A: two copies of the C282Y variant, most common cause of hereditary hemochromatosis, highest ferritin levels; lower serum manganese levels[ref][ref]
- A/G: one copy of C282Y, increased ferritin levels, hemochromatosis possible but less likely[ref], check to see if combined with H63D (below) – combo increases the risk of hemochromatosis; lower serum manganese levels[ref][ref]
- G/G: typical
Members: Your genotype for rs1800562 is —.
Check your genetic data for rs1799945 H63D (23andMe v4, v5; AncestryDNA):
- G/G: two copies of the H63D variant increase the risk of (mild) hemochromatosis, and increased ferritin levels; linked to lower serum manganese levels[ref]
- C/G: one copy of H63D, possibly higher ferritin levels, can cause hemochromatosis in conjunction with one copy of C282Y (above); linked to lower serum manganese levels[ref]
- C/C: typical
Members: Your genotype for rs1799945 is —.
Sources of iron:
Good sources of bioavailable iron include beef, liver, and seafood. The form of iron in plants is a little less bioavailable, so you may need more of it. Leafy green vegetables and dried fruits are good sources of plant iron.
Iron supplements are also available if you are deficient. Talk with your doctor, of course, if you have questions. Iron bisglycinate is the form that is gentle on the stomach and less likely to cause constipation.
Testing your iron levels:
The best way to know if you are deficient in iron is to get a blood test done. This is something that your doctor can order for you, or you can order your own blood test in the US in most states.
Gene-Diet interactions with congenital heart disease risk:
Congenital heart disease refers to a nynber of different types of structural defects in the heart that are present at birth. These structural problems can include problems with the heart valves, a hole in the heart wall, or even problems with blood vessels that have developed incorrectly. [ref]
Maternal diet plus genes:
A maternal diet that is high in smoked foods, fried foods, and barbequed foods increases the overall relative risk of congenital heart defects in the baby. The risk is exacerbated by a couple of genetic variants in the CBS and ADIPOQ genes.[ref]
Genetic connections with congenital heart disease:
CBS gene:
Check your genetic data for rs2851391 (23andMe v4, v5; AncestryDNA):
- T/T: increased risk of congenital heart disease in baby with poor maternal diet (fried foods, BBQ, and smoked foods)[ref]
- C/T: typical risk
- C/C: typical
Members: Your genotype for rs285139 is —.
Check your genetic data for rs234714 (23andMe v5):
- T/T: increased risk of congenital heart disease in baby with poor maternal diet (fried foods, BBQ, and smoked foods)[ref]
- C/T: typical risk
- C/C: typical
Members: Your genotype for rs234714 is —.
ADIPOQ gene:
Check our genetic data for rs2241766 (23andMe v4, v5; AncestryDNA):
- G/G: increased risk of congenital heart disease in baby with poor maternal diet (fried foods, BBQ, and smoked foods)[ref]
- G/T: typical risk
- T/T: typical
Members: Your genotype for rs2241766 is —.
Preeclampsia
Preeclampsia is a pregnancy complication involving high blood pressure and protein in the urine. In addition to being a serious health concern for the mother, preeclampsia is also a risk factor for preterm birth and growth restriction in the fetus.
I’m just hitting the highlights here on preeclampsia. This is a complex topic with many environmental and genetic risk factors.
Preeclampsia genetic connections:
ANRIL gene:
Check your genetic data for rs4977574 (23andMe v4, v5; AncestryDNA):
- G/G: lower risk of preeclampsia[ref]
- A/G: lower risk of preeclampsia
- A/A: typical
Members: Your genotype for rs4977574 is —.
Check your genetic data for rs1333048 (23andMe v4, v5; AncestryDNA):
- C/C: lower risk of preeclampsia[ref]
- A/C: lower risk of preeclampsia
- A/A: typical
Members: Your genotype for rs1333048 is —.
LRP8 gene: encodes APOE receptor 2, also called Low-density lipoprotein receptor–related protein 8
Check your genetic data for rs2297660 (23andMe v4, v5; AncestryDNA):
- T/T: in women with preeclampsia, reduced risk of low birth weight baby[ref]
- G/T: in women with preeclampsia, reduced risk of low birth weight baby
- G/G: typical
Members: Your genotype for rs2297660 is —.
CYP11B2: encodes aldosterone synthase, which is an essential part of blood pressure regulation
Check your genetic data for rs1799998 C344T (23andMe v5; AncestryDNA):
- A/A: typical
- A/G: slightly increased heart disease risk[ref]
- G/G: increased relative risk of hypertension in many different population groups (but not all)[ref][ref][ref][ref]; higher relative risk of preeclampsia[ref]
Members: Your genotype for rs1799998 is —.
Environmental factors that influence the baby
While I don’t have genetic connections included in this section, I wanted to bring in other research on pregnancy to be aware of.
ADHD and maternal acetaminophen:
Epidemiologic studies show an association between maternal acetaminophen (Tylenol is a name brand) use and ADHD rates. A study using umbilical cord blood metabolites and maternal acetaminophen use found that higher acetaminophen exposure was associated with a 2- to 3-fold increase in the risk of ADHD[ref]
Maternal glucocorticoid use during pregnancy:
A study (Dec 2024) examined glucocorticoid exposure in utero. The study included all babies born in Denmark between 1996 and 2016. The children were categorized based on their mothers’ autoimmune/inflammatory diseases and glucocorticoid use during pregnancy. The researchers also examined glucocorticoid use in the prevention of preterm births. Children born to mothers taking glucocorticoids were at a 30-50% increased relative risk of ADHD (or autism). [ref]
Schizophrenia:
Epidemiological studies link prenatal, maternal, and early-life infections to an increased risk of schizophrenia. Prenatal maternal infection with Toxoplasma gondii during pregnancy is linked in many studies to an increased risk of schizophrenia. A 2023 study using UK Biobank data showed that antibodies to T. gondii significantly increased the risk of developing schizophrenia.[ref][ref][ref][ref] T. gondi is the parasite that causes toxoplasmosis. This is one reason why pregnant women are told not to clean their cat’s litter box.
Phthalate exposure:
Phthalates are endocrine disruptors found in plastics, vinyls, and artificial fragrances (laundry detergent, lotions, air fresheners, etc). In pregnant women, higher phthalate metabolite levels are associated with lower free T4 levels as well as altered TSH/FT4 ratios. The conclusion was that “exposure to phthalates may interfere with the thyroid system during pregnancy.”[ref]
Fluoride:
Pregnant women pass about 60% of the concentration of fluoride in their bloodstream to the placenta at lower levels. At high concentrations, the placenta acts as more of a barrier to protect the fetus from high fluoride levels. Fluoride also passes through breast milk at low concentrations.[ref] Higher urinary levels of fluoride in the mothers during pregnancy resulted in an increased risk of neurobehavioral problems. [ref]
Talk with your doctor if you have any questions about what is right for you during your pregnancy.
Related articles and topics:
Histamine Intolerance: Genetic Report, Supplements, and Real Solutions