Key takeaways:
~ Genetic variants in the opioid receptor genes impact your ability to feel pain and to have pain blocked by opioid drugs.
~ Certain variants increase the relative risk of addiction to opioids.
How do opioid receptors work?
Pain helps us to learn not to do something, like touching a hot stove. But we have built-in ways to turn off the pain at some point. One way is through natural opioids the body makes as a way to regulate pain, reward, and addictive behaviors.
Genetic variants, though, mean that we don’t all feel the same amount of pain. Some feel pain more strongly or for longer times than others. These same variants also impact our reactions to opioid-based medications, impacting our susceptibility to opioid addiction.
There are three opioid receptors: mu, delta, and kappa (μ, δ, and κ).
They differ in how they bind with different types of opioid drugs and opioid molecules made in the body, but they all are somewhat similar and related.[ref]
Let’s look at all three of them in a little more detail.
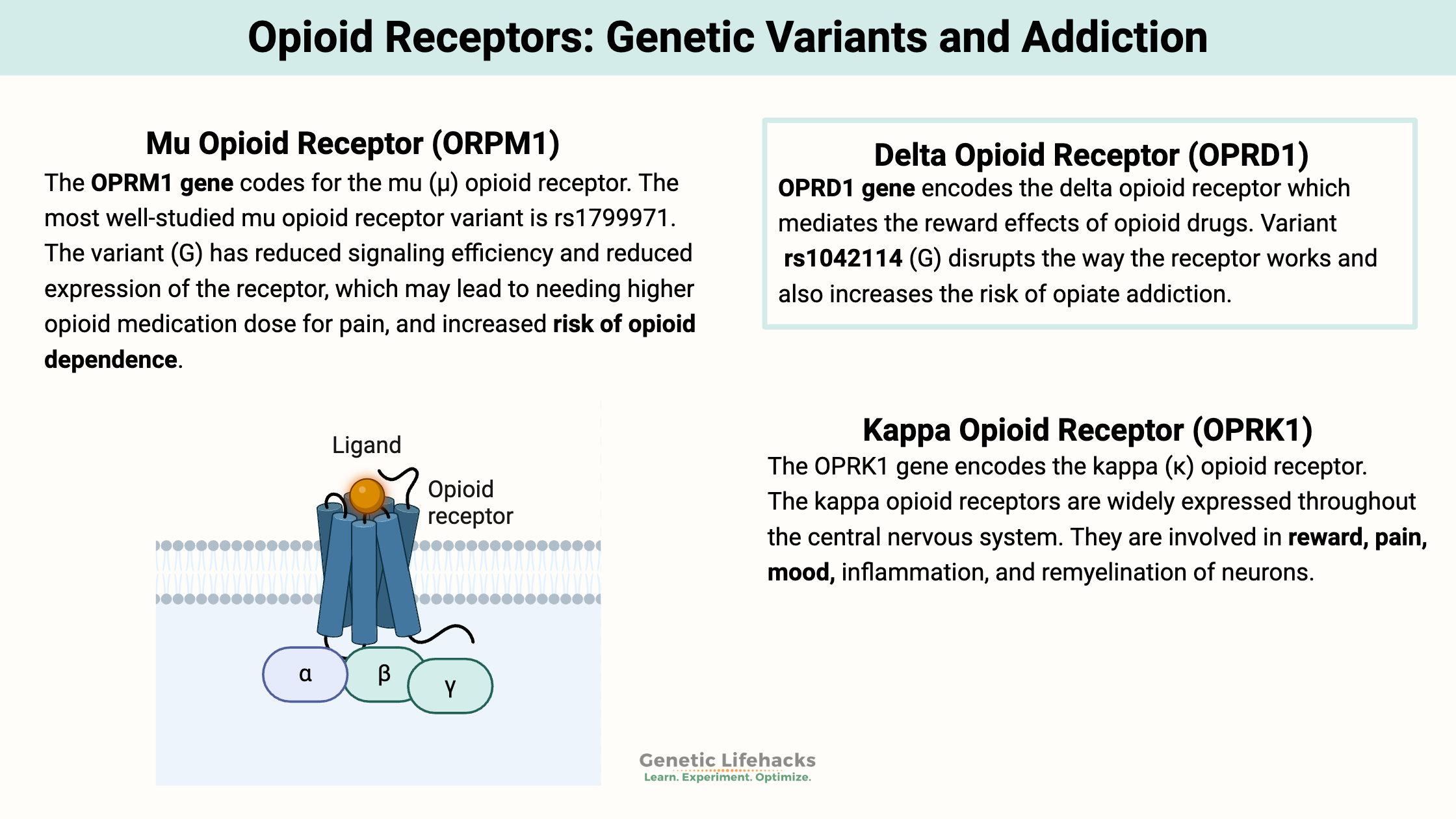
Mu Opioid Receptor (ORPM1):
The OPRM1 gene codes for the mu (μ) opioid receptor. These opioid receptors are located on the outer membranes of neurons. When something binds to that receptor, it triggers a chemical change that starts a cascade of events. This cascade leads to pain relief and feelings of pleasure or euphoria due to an increase in dopamine.
Diving a little deeper:
Mu opioid receptors are found in the neurons of the spinal cord, the brain, and the intestines.
To understand how mu-opioid receptors work, we need to talk about how the pain signal reaches the brain.
- Initiating the signal: Nociceptors are the receptors that initially signal to the brain that there is pain. For example, when you hit your thumb with a hammer, the nociceptors in your thumb will trigger a signal that it hurts.
- Relaying the signal: This initiates a multistep process to relay the signal, and then the brain also is involved in learning from the pain (e.g., don’t hit your thumb with a hammer).
- Reducing the signal: Activating the opioid receptors partially blocks the activation of the neurons that are relaying the pain signal from the nociceptor. This activation can come from substances naturally created in the body (endogenous ligands) or opioid drugs (exogenous ligands).
Delta Opioid Receptor (OPRD1):
The OPRD1 gene encodes the delta (δ) opioid receptor. The delta opioid receptor has been linked to mood regulation for anxiety and depression. [ref] It is unique in that it seems to regulate brain activity related to related to reward circuits, but without the problems associated with abuse. Activation of the delta opioid receptor is associated with having a positive effect on reducing anxiety.[ref]
The delta opioid receptors are found in the prefrontal cortex, which is the part of the brain involved in controlling impulsivity and drug seeking. These receptors can help to fine-tune GABA (inhibitory neurotransmitter) release.[ref]
Animal studies show that delta opioid receptor activation helps attenuate cognitive impairment and beta-amyloid production in an Alzheimer’s model. The activation of the delta opioid receptor inhibited HMGB1 secretion. HMGB1 is involved in DNA damage repair but also increases inflammation.[ref]
Related article: HMGB1 genes
Kappa Opioid Receptor (OPRK1):
The OPRK1 gene encodes the kappa (κ) opioid receptor. The kappa opioid receptors are widely expressed throughout the central nervous system. They are involved in reward, pain, mood, inflammation, and remyelination of neurons.[ref]
Dysregulation of the kappa receptors may have a role in depression and anxiety. Activation of the kappa opioid receptor is associated with dysphoria and a stress-like response.[ref]
Endogenous ligands of the opioid receptors:
Endogenous ligand is a fancy way of saying “stuff your body makes” – in this case, natural activators of the opioid receptors. The endogenous opioid peptides are derived from three precursors: proopiomelanocortin (POMC), proenkephalin, and prodynorphin.
β-endorphins are produced by the pituitary and hypothalamus to maintain homeostasis and reduce stress. β-endorphins can be released into the body and decrease pain through the neurons in the peripheral nervous system. They can also be released in different brain regions. When β-endorphins bind to the opioid receptor, this inhibits GABA, which then allows for a greater release of dopamine.[ref] Dopamine is associated with pleasure and reinforces learning, rewarding you.
Another natural opioid peptide that your body produces is enkephalin. This neuropeptide is found in the brain and the adrenal medulla, and it can bind to the mu or delta opioid receptor.[ref] Enkephalin reduces the production of substance P, which is the signaling molecule that transmits pain via your neurons.
Dynophorins are a group of molecules that bind to the kappa receptors and modulate neuronal excitability. They have a complex role in pain modulation by dampening the pain signal while also increasing sensitivity to pain in some situations.[ref]
The endogenous opioid peptides are derived from three precursors: proopiomelanocortin (POMC), proenkephalin, and prodynorphin.
Exogenous ligands (drugs that bind to the receptor):
Opium has been used for thousands of years for pain relief and to treat dysentery. It comes from the dried latex that is exuded from a cut on a poppy seed pod. Historically, opium has been smoked or made into a tincture called laudanum. Wars have been fought over it. The purification of opium into morphine was a historical breakthrough in that it allowed doctors to treat patients with a known dose rather than relying on the vagaries of the potency of raw opium. Merck Pharmaceutical Company was founded on the sale of morphine.[ref]
There are several important medications and illegal recreational drugs that bind to the mu opioid receptors that are derived from opium (called opiates) or are synthetic opioid receptor agonists (called opioids):
- Morphine
- Oxycodone, codeine, hydrocodone
- Fentanyl
- Heroin
- Methadone
- Buprenorphine (partial mu agonist)
When an opioid binds to the mu-opioid receptor, it reduces the excitability of the neurons by inhibiting GABA. GABA is an inhibitory neurotransmitter. In addition to decreasing pain, this also causes more dopamine to be released.
Naloxone and naltrexone are antagonists that bind to and block the mu receptor. Naloxone, or Narcan, stops the action of opioid drugs, and Narcan is used for opioid overdoses.
Kappa opioid receptor agonists include salvinorin A, which is a psychotropic derived from Salvia divinorum. Buprenorphine is a partial kappa receptor agonist used as a pain medication.
There are several delta opioid receptor drugs that are used in research. Buprenorphine is a partial delta agonist.
Opioid addiction:
Long-term use of opioids for pain can lead to tolerance, dependence, and addiction. Essentially, repeated activation of the mu opioid receptor leads to desensitization and then internalization of the receptor so that it is no longer on the surface of the cell. Long-term, this can lead to a reduced number of receptors, causing the need for higher doses to get the same pain relief.[ref]
The risk of opioid addiction (heroin or other opioids) is partially due to genetic vulnerability. Twin studies show 40 – 60% heritability for opiate addictions.[ref] Genetics isn’t the whole picture here, but it is important.
The studies on opioid addiction show varied results for the genetic variants associated with it. There are likely multiple genes involved as well as the many complex environmental factors.
A recent study in the journal Pain Physician found that patients with both dysfunctional CYP2D6 variants and OPRM1 variants were at the highest risk for opioid addiction (estimated to be 14% of people). Patients with ‘subnormal’ or somewhat impaired variants of CYP2D6 or OPRM1 were considered to be at medium risk (~48% of people). The rest, with functional CYP2D6 and OPRM1 variants, were considered at low risk. (~38% of the population). The study considered OPRM1 functionality on the rs1799971 variant is listed below. You can check your CYP2D6 variants here.
Genotype Report: Opioid Receptors
More to learn:
Related Articles and Topics:
Alcohol Addiction: Exploring the Genetic and Environmental Factors
References:
AL-Eitan, Laith N., et al. “Genetic Susceptibility of Opioid Receptor Genes Polymorphism to Drug Addiction: A Candidate-Gene Association Study.” BMC Psychiatry, vol. 21, no. 1, Jan. 2021, p. 5. BioMed Central, https://doi.org/10.1186/s12888-020-03006-z.
Cahill, Catherine, et al. “Fundamentals of the Dynorphins / Kappa Opioid Receptor System: From Distribution to Signaling and Function.” Handbook of Experimental Pharmacology, vol. 271, 2022, pp. 3–21. PubMed Central, https://doi.org/10.1007/164_2021_433.
Dalefield, Martin L., et al. “The Kappa Opioid Receptor: A Promising Therapeutic Target for Multiple Pathologies.” Frontiers in Pharmacology, vol. 13, June 2022, p. 837671. PubMed Central, https://doi.org/10.3389/fphar.2022.837671.
Hancock, Dana B., et al. “Cis-Expression Quantitative Trait Loci Mapping Reveals Replicable Associations with Heroin Addiction in OPRM1.” Biological Psychiatry, vol. 78, no. 7, Oct. 2015, pp. 474–84. PubMed Central, https://doi.org/10.1016/j.biopsych.2015.01.003.
Klein, Amanda H., et al. “Over-Expression of Mu-Opioid Receptors in Peripheral Afferents, but Not in Combination with Enkephalin, Decreases Neuropathic Pain Behavior and Enhances Opioid Analgesia in Mouse.” Anesthesiology, vol. 128, no. 5, May 2018, pp. 967–83. PubMed Central, https://doi.org/10.1097/ALN.0000000000002063.
Pasternak, Gavril W., and Ying-Xian Pan. “Mu Opioids and Their Receptors: Evolution of a Concept.” Pharmacological Reviews, vol. 65, no. 4, Oct. 2013, pp. 1257–317. PubMed Central, https://doi.org/10.1124/pr.112.007138.
Ruano, Gualberto, and Jonathan A. Kost. “Fundamental Considerations for Genetically-Guided Pain Management with Opioids Based on CYP2D6 and OPRM1 Polymorphisms.” Pain Physician, vol. 21, no. 6, Nov. 2018, pp. E611–21.
Valentino, Rita J., and Nora D. Volkow. “Untangling the Complexity of Opioid Receptor Function.” Neuropsychopharmacology, vol. 43, no. 13, Dec. 2018, pp. 2514–20. PubMed Central, https://doi.org/10.1038/s41386-018-0225-3.
—. “Untangling the Complexity of Opioid Receptor Function.” Neuropsychopharmacology, vol. 43, no. 13, Dec. 2018, pp. 2514–20. www.nature.com, https://doi.org/10.1038/s41386-018-0225-3.
Xu, Yuan, et al. “Delta-Opioid Receptor Signaling Alleviates Neuropathology and Cognitive Impairment in the Mouse Model of Alzheimer’s Disease by Regulating Microglia Homeostasis and Inhibiting HMGB1 Pathway.” Alzheimer’s Research & Therapy, vol. 17, no. 1, Feb. 2025, p. 35. PubMed, https://doi.org/10.1186/s13195-025-01682-1.
Zhang, H., et al. “The OPRD1 and OPRK1 Loci in Alcohol or Drug Dependence: OPRD1 Variation Modulates Substance Dependence Risk.” Molecular Psychiatry, vol. 13, no. 5, May 2008, pp. 531–43. PubMed Central, https://doi.org/10.1038/sj.mp.4002035.
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.