Key takeaways:
~ Acute pancreatitis is a painful, severe inflammation of the pancreas.
~ Recent research shines new light on the underlying mechanisms that cause pancreatitis.
~ Genetic variants increase susceptibility to pancreatitis, and understanding these genetic pathways may help to prevent a recurrence.
~ Targeting the right pathways with diet and natural supplements may help.
This article takes a deep dive into pancreatitis, chronic pancreatitis, and exocrine pancreatic insufficiency. You may want to bookmark it now so that you can come back to it later. Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
What is pancreatitis?
Acute pancreatitis is an inflammation of the pancreas that causes a sudden onset of upper abdominal pain, often accompanied by nausea. Blood tests show elevated serum amylase and lipase levels.[ref]
The pancreas is an organ located in the upper abdomen. It produces and releases enzymes and hormones needed to digest food, and a small part of the pancreas also produces and releases insulin.
The part that releases the digestive enzymes is called the exocrine pancreas, while the insulin-releasing part is called the endocrine pancreas. The exocrine part of the pancreas makes up about 96% of the pancreas.
Digestive enzymes secreted by the pancreas include amylase (which breaks down carbohydrates), proteases (which break down proteins), and lipases (which break down fats).
These digestive enzymes are made in the acinar cells and released into the pancreas.
According to the Pancreas Foundation, symptoms of pancreatitis include:[ref]
- Gradual or sudden pain in the upper abdomen
- Abdominal pain can be severe, constant, and may be worse after eating
- Nausea and vomiting
- Fever, rapid pulse
Pancreatitis Severity:
Pancreatitis can be classified as mild, moderate, or severe.
The mild form, while still painful, is self-limiting and resolves. Severe acute pancreatitis, which accounts for ~20% of cases, can cause organ failure that persists as well as causing other organ complications and even death.
Here’s a breakdown of pancreatitis classification, adapted from PMC9874226:
Mild acute pancreatitis (MAP)
|
Moderately severe acute pancreatitis (MSAP)
|
Severe acute pancreatitis (SAP)
|
What is happening in acute pancreatitis?
In pancreatitis, there is a systemic inflammatory response to damage to pancreatic cells, which is most commonly due to trypsin causing autodigestion in the pancreatic tissue.[ref]
In other words, you are breaking down and digesting your own pancreas, and the cell damage causes your immune system to respond.
The cells that are initially damaged are pancreatic acinar cells and fat cells. Acinar cells are the exocrine cells that release pancreatic enzymes to break down food.[ref] The proteases – protein-metabolizing digestive enzymes – actually break down the proteins that make up your pancreatic tissue.
But what initiates trypsin activation in the pancreas? First, there is some kind of triggering event that causes a ‘pancreatic insult’ — or genetics is involved.
Triggers of pancreatitis include:
- Gallstones lodged in the pancreatic duct can be one cause of acute pancreatitis. This is called biliary pancreatitis and blocks the release of pancreatic enzymes.
- Alcohol abuse is another common cause. Ethanol damages the cells in the pancreas.
- Pathogens, such as viruses or bacteria, can cause pancreatitis.[ref]
- Drugs or toxins can also trigger pancreatitis.
- Trauma or injury to the pancreas is another possibility.
- High triglycerides (>1,000 mg/dL) or high calcium levels can be a trigger. [ref]
- Anabolic steroid use is also a rare cause of pancreatitis.
- Autoimmune pancreatitis is another cause.
- Sphincter of Oddi dysfunction[ref]
- Genetic mutations can cause hereditary pancreatitis.
Autodigestion and Inflammation
The pancreas, specifically the acinar cells, normally release digestive proteases (protein-digesting enzymes) in an inactive, precursor form. These inactive proteases are flushed into the upper part of the small intestine in a fluid rich in sodium bicarbonate.
In the duodenum (upper small intestine), trypsinogen, the precursor for the protein-digesting enzyme trypsin, becomes activated by certain enzymes produced there.
However, trypsinogen can also be autoactivated by trypsin. Researchers believe that this autoactivation of trypsinogen by trypsin may sometimes occur in the pancreas, causing pancreatitis. This premature activation of trypsinogen doesn’t usually occur because of activation inhibition by an enzyme called SPINK1.[ref] (Mutations in SPINK1 can cause hereditary pancreatitis.)
Trypsin’s job is to cut apart proteins, which is essential for digesting foods containing protein. But if trypsin is activated and formed inside the pancreas, it can also cut apart the proteins that make up pancreatic cells.
Autodigestion of pancreatic tissue causes cell death, which triggers an innate immune response and inflammatory cascade. This is a natural response of the innate immune system, which not only recognizes foreign pathogens but also is activated by damaged cells. This is called a sterile inflammatory response.
In people with severe acute pancreatitis, this innate immune response is profoundly deranged compared to mild or moderate acute pancreatitis. In severe pancreatitis, the sterile inflammation is excessive and systemic, causing damage to other organs, which in turn drives more of an immune response to the new damage.[ref] According to one study, the difference between mild or moderate pancreatitis vs. severe pancreatitis “depends on whether the inflammatory response resolves or amplifies, leading to multi-organ failure.”[ref]
More to the pancreatitis story than trypsin:
While the activation of trypsin in the pancreas is a driving factor in initiating the damage in pancreatitis, it may not be the only causative factor.
Trypsin isn’t the only culprit:
Surprisingly, animal studies show that pancreatitis can be induced even in animals that don’t produce trypsinogen (or trypsin).[ref]
One study explains: “NF-κB activation that happens parallel and independent to trypsinogen activation can still drive the development of the acute or chronic pancreatitis even in absence of trypsin.”[ref]
This has led researchers to believe that pancreatitis can also be driven by multiple factors, including the translocation of gut bacteria, viruses, or fungi into the pancreas.[ref]
In addition, a recent study showed that cell death in the acinar cells is caused by endoplasmic reticulum stress and can be independent of trypsinogen activation. Inhibition of endoplasmic reticulum (ER) stress can stop pancreatic tissue damage.[ref]
Other researchers point to alterations in autophagy in addition to inflammation as a cause of pancreatitis.[ref]
Let’s dig deeper into the research on inflammation and then take look at autophagy and the gut microbiome:
Inflammation in Pancreatitis:
In the inflammatory cascade that occurs with pancreatitis, damaged cells activate pathways that increase inflammation, including HMGB1, TLR4 (toll-like receptor 4), TLR9, and heat shock protein 70. This then activates IL-1B, NLRP3, and IL18.[ref]
Related articles for more details: HMGB1, NLRP3, Heat shock proteins
TLR4 is a key player in acute pancreatitis. TLR4 (toll-like receptor 4) is a pattern recognition receptor protein that activates more inflammatory pathways (NF-kB) when it is activated by cell damage or by a pathogen.
Researchers have found that reducing the Tlr4 gene expression in mice significantly reduces cell death in the pancreas in a mouse model of pancreatitis.[ref] TLR4 is found at higher levels in people with acute pancreatitis, compared to healthy people.[ref] Genetic variants that increase TLR4 are associated with increased susceptibility to pancreatitis (see genotype report).
Neutrophil extracellular traps (NETs) on the surface of neutrophils (a type of white blood cell) are also activated in response to the inflammatory cytokines or to the damaged particles. NETs are also implicated in autoimmune diseases and long Covid, but in bacterial infections, NETs are immunoprotective, trapping bacteria for removal.
In a mouse model of pancreatitis, mice that couldn’t form NETs had decreased pancreatitis severity and increased survival. In this study, chloroquine, which is a drug sometimes used in autoimmune diseases, decreased the propensity to form NETs.[ref]
Heat shock proteins are chaperone proteins that are activated in response to cellular stress. In the pancreas, heat shock protein 70 protects acinar cells during inflammation. HSP70 levels are lower in people with severe acute pancreatitis and depressed in non-survivors. [ref]
Heat shock proteins may do more than just protect the cells during inflammation. They may also play a role in preventing trypsinogen activation. In mice that are genetically altered not to produce Hsp70, the mice had spontaneous trypsinogen activation.[ref][ref]
Cytokine levels are also elevated in people with pancreatitis, likely due to the activation of TLRs and inflammatory cascades. In people with pancreatitis, TNF-alpha, interferon-gamma, IL-1, IL-2, IL-6, IL-8, and IL-18 are elevated in direct proportion to the severity of the disease.[ref] Genetic variants that increase TNF, IL1, IL6, and IL8 are associated with increased susceptibility to acute pancreatitis . (see Genotype Report section)
Monocytes, macrophages, and lymphocytes are activated in part by MCP-1 (CCL2 gene), which research has shown to play an important role in the development of acute pancreatitis. Blocking MCP-1 has been shown in animal studies to attenuate the severity of pancreatitis.[ref] (see Genotype Report section)
It may be more than just the inflammation that causes pain and damage in pancreatitis. Researchers have found that TNF-alpha prematurely activates proteases in the pancreas.[ref] This may mean that elevated inflammatory cytokines can add to the cell damage in the pancreas by activating proteases, which then cause more cell damage.
Histamine is released from mast cells as part of the innate immune response. It has been discovered that people with painful chronic pancreatitis have more mast cells in their pancreas. In the acute, early phase of pancreatitis, histamine levels throughout the body rise due to the production of histamine from pancreatic mast cells. The large number of mast cells in the pancreas degranulate and release histamine as well as inflammatory mediators in acute pancreatitis.[ref]
Genetic variants in many of the above inflammatory pathways are linked to increased susceptibility to pancreatitis. Members can check their data in the Genotype Report section below to see where their genetic susceptibility lies.
Autophagy playing a causal role in pancreatitis?
Autophagy is the cellular process of breaking down and recycling cellular waste, such as damaged organelles. In autophagy, cells form lysosomes, which wall off the damaged organelles and then break them down with proteases and high pH. The amino acids and compounds that make up the organelles are then re-used by the cell to make new proteins.
In pancreatitis tissue samples, researchers can see that the autophagy mechanisms aren’t functioning as they should. This then leads to the question of whether pancreatitis causes problems with the completion of the autophagy process — or whether impaired autophagy is causing pancreatitis. The chicken or the egg question.
Animal studies show us that impaired autophagy can cause pancreatitis. When researchers knock out some of the key autophagy genes, the animals develop pancreatitis.
In animal models of pancreatitis, excessive intracellular calcium levels can cause ROS in the acinar cells. The excess calcium ions directly damage mitochondria in the cells. Autophagy then normally takes care of the damage in the cell. However, if autophagy is impaired in the pancreatic acinar cells, it may not be able to take care of the intracellular damage — leading to the inflammatory cascade.[ref]
Additionally, autophagy is important in tamping down inflammation due to tissue damage. So if the pancreas has been damaged (e.g. a gallstone got stuck or too much alcohol), a decreased ability of the cells to complete autophagy could lead to an increased inflammatory response to the damaged cells.[ref]
Viral and bacterial infections that cause pancreatitis:
Approximately 10% of acute pancreatitis cases are thought to be caused by viruses or bacteria.[ref]
Coxsackie B:
Injecting mice with the Coxsackie B virus is one way that researchers create an animal model of pancreatitis. Humans can also develop acute pancreatitis and chronic pancreatitis from Coxsackie B infection.[ref]
The Coxsackie Group B virus, a type of enterovirus, can infect the pancreas and persist in some people. Coxsackie B viral infections are thought to be a cause of type 1 diabetes, by infecting the pancreatic islet cells. Type 1 diabetes is an autoimmune disease in which the immune system attacks the pancreatic cells.[ref][ref]
While most people clear the virus quickly, some people with coxsackie B infections have long-term, chronic replication of the virus in certain cell types, such as the pancreas. Changes to the pancreatic duct cells are seen in persistent viral infections of the pancreas.[ref]
Other viruses:
Mumps and HIV infection can also cause pancreatitis. In rare cases, cytomegalovirus infection has also been shown to cause pancreatitis.[ref] Additionally, the SARS-CoV-2 virus can infect the pancreas.[ref]
Bacterial and other infectious causes:
Several different bacterial causes of acute pancreatitis have been identified. For example, Mycoplasma pneumonia can cause pancreatitis, as can typhoid and certain Salmonella bacteria. [ref] Brucellosis can also cause pancreatitis.[ref] Fungi (Aspergillus) and parasites (Toxoplasma, Cryptosporidium, Ascaris) can also cause pancreatitis.[ref]
Gut Microbes and Pancreatitis:
Severe acute pancreatitis can lead to systemic inflammation and necrotic infection of the pancreas. Researchers have found that the bacteria causing necrotic pancreatic infections come from the intestinal microbiome.
This led researchers to investigate the role of the gut microbiome in the development of acute pancreatitis.
Changes in the gut microbiome are seen in pancreatitis patients when compared to a healthy control group. For example, beneficial bacteria such as bifidobacteria are decreased in patients with chronic pancreatitis or recurrent acute pancreatitis.[ref] Animal studies show that there is a significant decrease in gut microbial diversity in pancreatitis. [ref]
The microbes in your gut interact with the intestinal mucosal barrier, which in turn interacts with your immune system. For example, the abundance of certain gut bacteria will elevate IL-6 levels.
In addition, there is a two-way communication between the gut and the pancreas. The production of certain peptides produced by the endocrine part of the pancreas is controlled by short-chain fatty acids, such as butyrate, produced in the gut microbiome. Antimicrobials that shape the gut microbiome are secreted by the exocrine part of the pancreas. [ref]
One study explains that bacterial overgrowth and leaky gut interact with pancreatic inflammation “allowing translocation of bacteria and toxins to the pancreas.” Additionally, about one-third of chronic pancreatitis patients are also diagnosed with small-intestinal bacterial overgrowth (SIBO).[ref]
In a case-control study of hospitalized patients with acute pancreatitis, the pancreatitis patients had a higher abundance of sulfidogenic bacteria in their intestines and higher serum hydrogen sulfate levels. [ref]
Animal studies show that antibiotic-treated or germ-free mice have significantly less damage from experimentally induced pancreatitis.[ref]
Interestingly, the oral microbiome is also different in pancreatitis patients.[ref]
Drugs linked to pancreatitis:
Alcohol is by far the biggest risk factor for pancreatitis. However, other drugs can also trigger pancreatitis in 1-5% of cases.[ref][ref]
The diabetes and weight loss drugs liraglutide and semaglutide (Wegovy, Ozymic) are associated with an increased incidence of acute pancreatitis. [ref][ref][ref][ref][ref]
SSRI use increases the relative risk of pancreatitis (OR=1.7).[ref][ref] Serotonin is involved as a neurotransmitter in the release of pancreatic enzymes.
Other drugs include mesalamine, azathioprine, l-asparaginase, metronidazole, and others.[ref][ref]
Autoimmune Pancreatitis:
Another rare cause of pancreatitis is an autoimmune trigger.
Doctors have known for some time that chronic pancreatitis can be autoimmune in nature. Recently, researchers have classified autoimmune pancreatitis into two types. Type 1 autoimmune pancreatitis is more common and is a part of IgG4-Related Disease.[ref][ref] Type 2 autoimmune pancreatitis is relatively rare and described as idiopathic duct-centric pancreatitis.
IgG4-Related Disease is usually due to elevated systemic IgG4 antibodies which cause fibroinflammation in certain organs, including fibrosis and inflammation of the pancreas. The fibrosis causes diffuse swelling of the pancreas and symptoms of chronic pancreatitis. Acute pancreatitis symptoms are uncommon in people with autoimmune pancreatitis.
Sphincter of Oddi Dysfunction: A Mechanical Cause of Pancreatitis
The Sphincter of Oddi is the valve that controls the flow of bile and pancreatic enzymes into the stomach. Dysfunction of the Sphincter of Oddi can allow pancreatic enzymes to flow back into the pancreas and cause pancreatitis. The Sphincter of Oddi is controlled by cholecystokinin (CCK) release in response to a meal, and it is also affected by other gastrointestinal peptides such as gastrin, secretin, motilin, and octreotide. The vagus nerve is also involved in controlling the sphincter of Oddi. [ref]
Sphincter of Oddi dysfunction can be due to either a spasm or relaxation of the valve at the wrong time. It can be a cause of chronic pancreatitis, or it can cause gallbladder pain.
A major risk factor for sphincter of Oddi dysfunction is having gallbladder removal surgery. One study found that 10-20% of patients had pain six months after gallbladder surgery, and up to half of these patients met the diagnostic criteria for sphincter of Oddi dysfunction. Another risk factor for sphincter of Oddi dysfunction is hypothyroidism.[ref]
Sphincter of Oddi dysfunction can be caused by certain medications, including opioids.
Chronic Pancreatitis:
You can think of pancreatitis as a disease continuum that includes an acute pancreatitis episode, recurrent acute pancreatitis, and then chronic pancreatitis. Researchers note that for people who do not drink alcohol, genetic risk factors drive the recurrent and then chronic aspects of pancreatitis.[ref]
Chronic pancreatitis is usually diagnosed in someone who has previously had acute pancreatitis and continues to have recurrent episodes of pancreatitis. This can lead to a progressive inflammatory disorder and pancreatic gland destruction. Chronic pancreatitis patients may have periods of remission and periods when the inflammation flares up.[ref] [ref]
Chronic pancreatitis often has a hereditary component, especially when it begins in people younger than age 35 who aren’t alcoholics. Genetic mutations in genes related to trypsinogen degradation, trypsin inhibition, calcium transport, and cystic fibrosis are associated with to hereditary chronic pancreatitis. (more in the genotype report)[ref]
Symptoms of chronic pancreatitis can include periodic bouts of nausea, vomiting, diarrhea, abdominal pain, oily or fatty stools that smell foul, and eventual weight loss.[ref] However, not everyone with chronic pancreatitis has painful symptoms, with one study showing that 12% of chronic pancreatitis patients were painless.[ref]
Both chronic and acute pancreatitis increase the risk of pancreatic cancer, with the highest risk within the first three years of diagnosis.[ref][ref] Additionally, having had an episode of acute pancreatitis more than doubles the risk of developing diabetes within the next five years.[ref]
While mutations related to trypsin are usually the cause of chronic pancreatitis, a recent animal study showed that repeated activation of NF-kB (inflammatory pathway) was enough to cause chronic pancreatitis, even without the aberrant activation of trypsin.[ref]
What is exocrine pancreatic insufficiency (EPI)?
Acute and chronic pancreatitis can lead to exocrine pancreatic insufficiency (EPI).
Exocrine pancreatic insufficiency is pretty much what it sounds like – reduced pancreatic juice and digestive enzymes. In particular, lipase, which is the enzyme that breaks down fats, tends to be reduced, causing problems with digesting fatty foods.
Symptoms:
EPI can cause foul-smelling and fatty stools, diarrhea, bloating, and gas. The term maldigestion is often used and fairly descriptive.[ref]
Causes:
Chronic pancreatitis or repeated bouts of acute pancreatitis can lead to a loss of the acinar cells that produce digestive enzymes. Fibrosis of the pancreas due to repeated damage leads to reduced lipase secretion.[ref]
Approximately 60-90% of chronic pancreatitis patients have developed EPI after 10 years. Of people who have had acute pancreatitis, about 20% end up with EPI. Alcohol-related pancreatitis is also a common cause of EPI. People with cystic fibrosis also have a high risk of pancreatic damage and pancreatic insufficiency. [ref]
Age is the biggest risk factor for EPI:
Like many things in the body, the pancreas is affected by aging. Researchers estimate that fibrosis and atrophy of the pancreas cause moderate to severe EPI in 15% of people over the age of 70. In people over the age of 80, it is estimated that approximately 30% are likely to meet the clinical criteria for pancreatic insufficiency.[ref]
What causes pancreatic enzyme release?
When looking at pancreatic enzyme deficiency, much of the focus is on the damaged pancreatic cells that are not functioning well. But that’s not the whole picture for everyone.
Let’s take a look at what normally is going on with pancreatic enzyme release.
The release of pancreatic enzymes is also regulated by factors outside of the pancreas.
- pH matters here. In the upper small intestine, the optimal pH for pancreatic enzyme release is between 7 and 8.
- The vagus nerve plays is important in the release of pancreatic enzymes. It is stimulated by stomach stretching and by certain gastric peptides, such as CCK. (CCK also acts directly on pancreatic cells.)
- GABA is the major inhibitory neurotransmitter. Animal studies show that the microinjection of a GABA-A receptor antagonist (blocking the GABA-A receptor) increases pancreatic exocrine enzymes.
- Serotonin also affects pancreatic enzyme release. Activation of the serotonin receptor inhibits pancreatic insulin release. [ref]
Serotonin: While most people think of serotonin in terms of mood, it is also an essential neurotransmitter throughout the body, including driving intestinal motility and pancreatic functions.[ref]
Calcium ions serve as second messengers, relaying the message to the acinar cells to release pancreatic enzymes. One way animal models of pancreatitis are created by giving the animals compounds that increase calcium signaling in the acinar cells. Modulating calcium release can then ameliorate experimental-induced pancreatitis. [ref]
Magnesium can replace calcium in the acinar cells, and replacing calcium with magnesium can reduce the overactivation of acinar cells. Studies show that magnesium deficiency is very common in pancreatitis. Moreover, increasing magnesium in pancreatic cells can reduce calcium spikes and trypsin release. [ref][ref]
The good news is that pancreatic enzymes are readily available either by prescription or as an OTC supplement. More on this in the Lifehacks section below.
Testing for EPI:
Exocrine pancreatic insufficiency is usually diagnosed based on a stool test that looks for fat content and elastase-1 levels. This is something that your doctor can order, or you can order it yourself online (one source for the test is Ulta Lab Tests).
Is it IBS or EPI?
A study of people diagnosed with irritable bowel syndrome (IBS) found that about 6% of them actually had exocrine pancreatic insufficiency instead of IBS, according to their stool tests.[ref]
Genetics: Hereditary Pancreatitis vs. Susceptibility to Pancreatitis.
The genetic component of pancreatitis can be roughly divided into two areas: common variants that increase susceptibility to acute pancreatitis and then rare mutations that cause hereditary chronic pancreatitis.
Understanding your genetics can help you figure out which pathways to target.
Let’s start with the rare ones…
Hereditary pancreatitis:
Rare mutations cause hereditary pancreatitis, which can start at a young age. These types of mutations are changing the function of a gene in a big way.
I mentioned above that the inactive trypsinogen is formed in the pancreas and then converted into the active trypsin form in the intestines.
Trypsinogen is encoded by the PRSS1 and PRSS2 genes. Mutations in PRSS1 alter the trypsinogen protein so that it is more likely to be autoactivated in the pancreas, forming trypsin and causing hereditary pancreatitis.
SPINK1 is the enzyme that stops the activation of trypsinogen in the pancreas. Similarly, mutations in the SPINK1 gene can also cause hereditary pancreatitis due to easier autoactivation of trypsinogen. [ref]
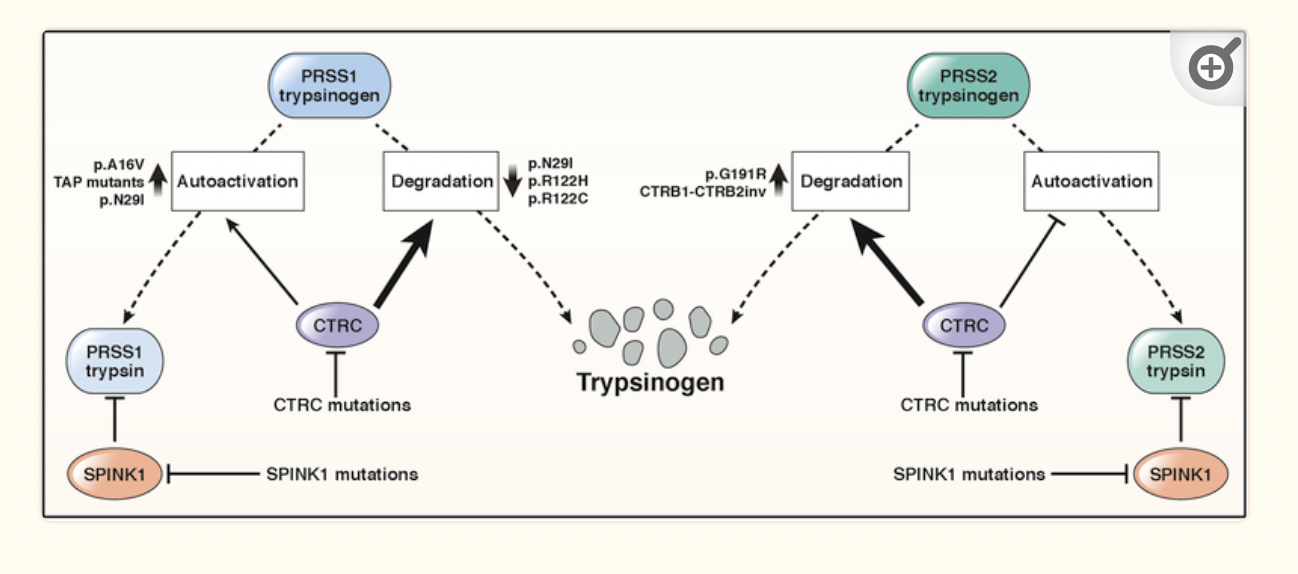
Mutations in the CFTR gene that cause cystic fibrosis can also cause pancreatitis. CFTR is involved in the way sodium bicarbonate is secreted along with the pancreatic digestive enzymes. Not everyone with cystic fibrosis mutations will end up with chronic pancreatitis, but it is a risk factor. [ref] Just carrying one copy of a CFTR mutation doesn’t usually cause any symptoms of cystic fibrosis, and carriers are usually unaware that they have the mutation. In one study of 16 people with idiopathic chronic pancreatitis, genetic testing showed that one-third of the patients carried a CFTR mutation.[ref]
Common genetic polymorphism:
The genetic polymorphisms associated with increased susceptibility to pancreatitis are all much more common in the population.
Several of the identified pancreatitis susceptibility genes are related to inflammatory cytokines or the immune response. An overactive immune response to the cell damage caused by some trypsin activation in the pancreas can increase the risk of pancreatitis.
For example, animal studies show that elevated soluble TNF-alpha can induce premature protease activation, which then causes cell death in the pancreas.[ref] Essentially, a little premature trypsin activation can snowball with inflammation and TNF-alpha causing more trypsin activation.
You’ll see in the Genotype Report section below that TLR4 variants impact susceptibility, along with IL-8 and IL-1B variants. These immune system genes are important in protecting us from pathogens, and a robust response can be an advantage when fighting off a virus or bacteria. However, an excessive inflammatory response is a disadvantage when it comes to susceptibility to pancreatitis.
Pancreatitis Genotype Report:
Lifehacks: Research-backed treatments for pancreatitis
Basics for people with recurrent acute or chronic pancreatitis:
- Don’t drink alcohol.
- Don’t smoke.
- Eat a healthy diet.
I’d like to point out here that the pancreas can regenerate after a bout of acute pancreatitis.[ref] With that in mind, let’s get into the details about supplements and diet for healing and preventing pancreatitis. I’ll also include clinical trials for pancreatitis treatment.
Supplements and dietary considerations:
There are many websites that give advice on what to eat when you have pancreatitis. To be honest, the advice is often contradictory – from eating 4-6 meals a day to fasting and eating only one meal a day. Some recommend avoiding dairy products, while others say to drink low-fat milk every day. Some sites say to eat starchy foods along with protein, while others say to avoid protein…
Almost all say to avoid eating a lot of fat if you have recurrent or chronic pancreatitis.
Everyone is unique, and your ideal diet is likely to differ depending on whether you have active damage that needs healing, insufficient enzyme production, etc. You may find that starting with a diet of easily digestible fruits and cooked vegetables and then adding in lean protein sources may be a wise way to go.
Research on 16 natural supplements and vitamins for pancreatitis:
Natural supplements may also help with healing from pancreatitis and preventing recurrence.