Key takeaways:
~ Lyme disease is caused by bacteria transferred by tick bites.
~ For some, the illness is limited and resolves easily, but for others, chronic and debilitating symptoms can last for years.
~ Genetic variants are part of why Lyme disease affects people differently. Your genes also play a role in how well antibiotics work to cure Lyme.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
Background on Lyme Disease:
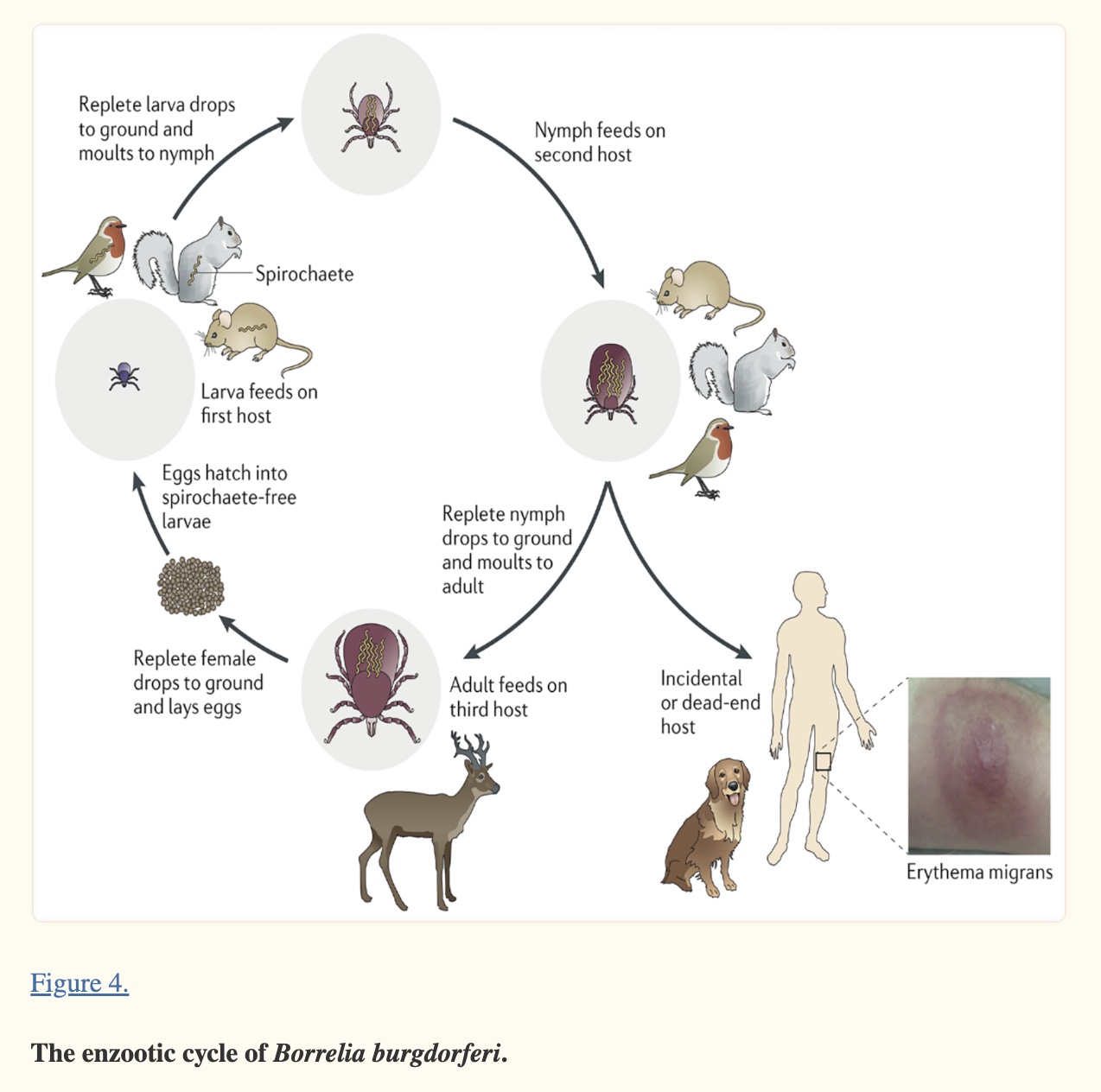
Lyme disease affects ~476,000 people a year in the US. While initially only in the Northeast and upper Midwest, ticks carrying Lyme disease are now found in almost all states.[ref][ref]
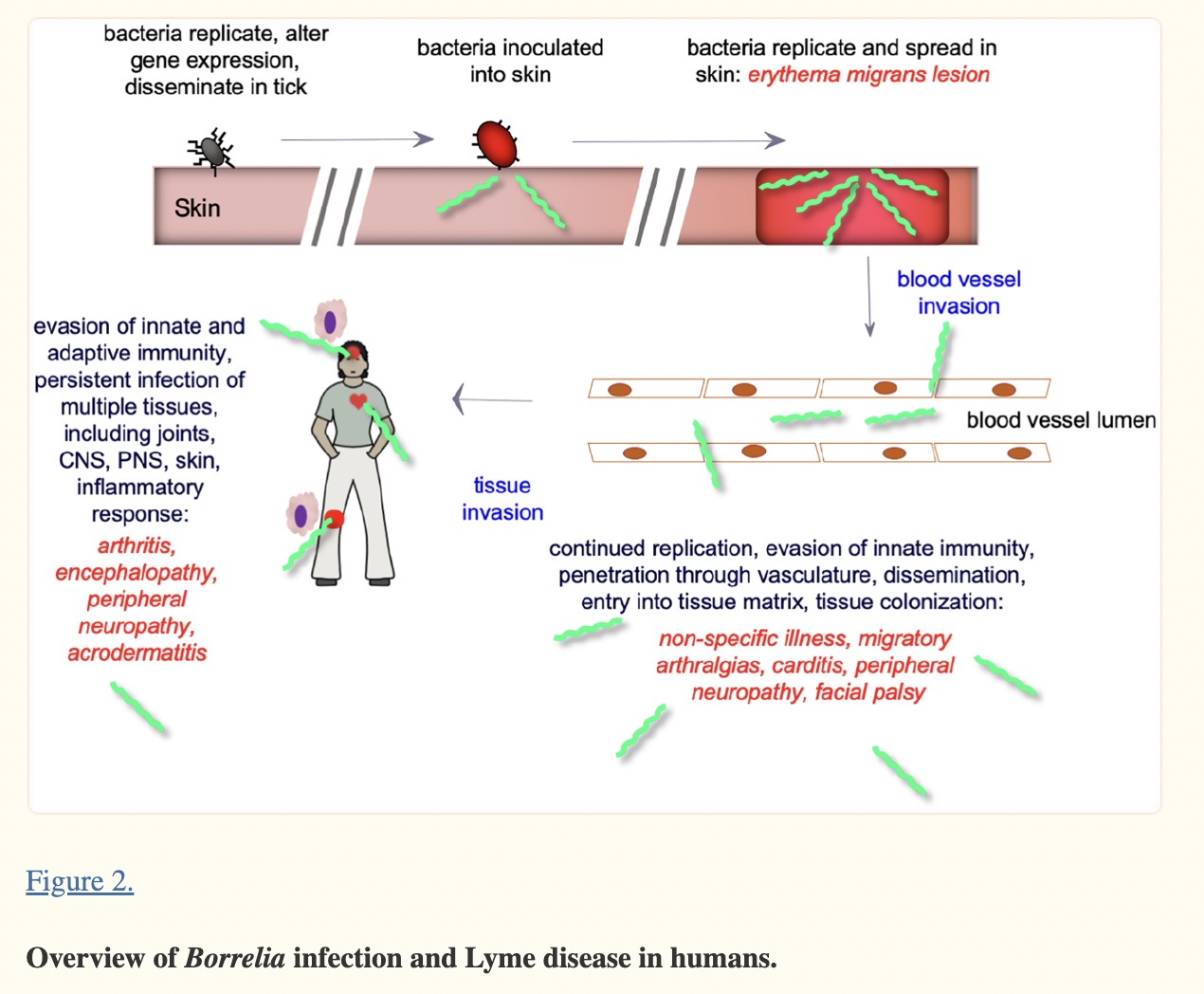
In North America, Lyme disease is caused by Borrelia burgdorferi, a bacterium carried by black-legged (deer) ticks, or a combination of Borrelia plus other microbes.
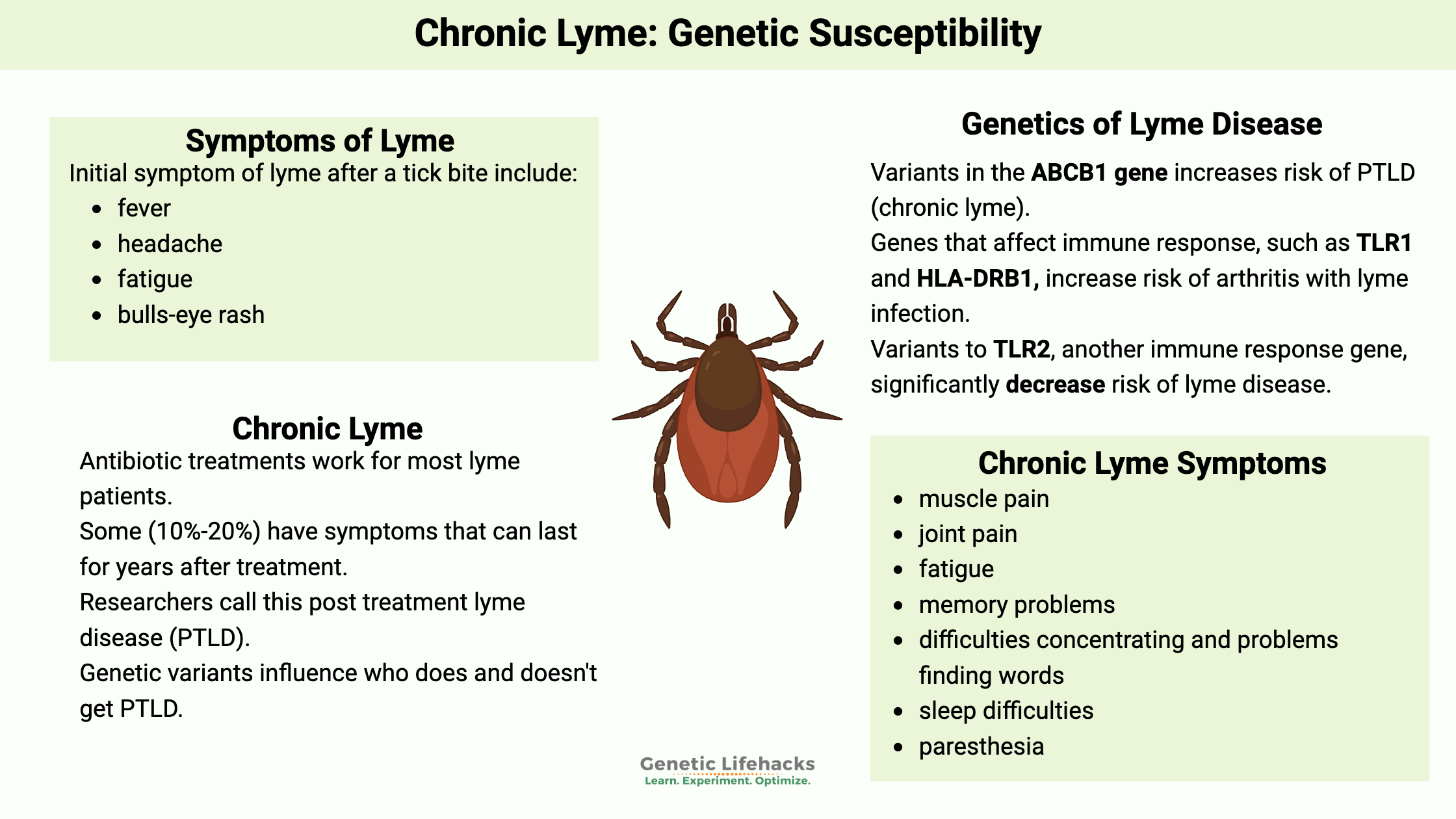
Initial symptoms of Lyme include:
- fever
- headache
- fatigue
- bulls-eye rash (erythema migrans) – sometimes
The CDC’s data on Lyme disease dates back to 1991, but reports of Lyme disease go back to the mid-1970s. The number of cases has grown steadily since the early ’90s, and the areas where the disease is found have spread across the US and Canada.[ref]
In Europe, Lyme disease is also present and sometimes called Borreliosis. Cases can involve other species of Borrelia, including B. afzelii, B. garinii, and B. valaisiana.[ref][ref]
The Borrelia species are a type of bacteria known as a spirochete. Our immune system has difficulty recognizing this bacterial spirochete because the surface proteins continually change, which keeps the pathogen one step ahead of the acquired immune response.[ref] Of note, there are genetic differences between historically known Borellia sp and the Borellia species that cause Lyme disease. Researchers in 2018 called for the genus to be separated and for the Lyme-causing species to be classified into a new genus, Borreliella.[ref] I’m sticking to Borrellia in this article because that is how most people still know it, but know that current research may refer to Borreliella burgdorferi.
Can Lyme disease be completely cured?
Several weeks of antibiotic treatment will cure Lyme disease for most people.[ref]
Unfortunately, not everyone reacts the same way to Lyme disease, and some have continuing symptoms long after the initial antibiotics.
Genetic variants can cause some people’s immune systems to act differently towards the Borrelia species, and other genetic variants can influence how well antibiotics work within your cells.
Additionally, some ticks are infected with multiple bacterial and/or viral pathogens.
What is chronic Lyme disease?
People use the term chronic Lyme to indicate they still have symptoms such as fatigue and brain fog after undergoing antibiotic treatment for Lyme disease.
Symptoms can sometimes last for years, significantly impacting quality of life. Chronic Lyme can be hard to diagnose, with some sufferers seeing multiple physicians and going years without a definitive diagnosis.
Controversy: There used to be controversy regarding the diagnosis of chronic Lyme because it was initially dismissed by some physicians who called it psychosomatic. Even recent articles, such as this one, use terminology such as “supposed symptoms” and claim that the “medical establishment maintains that there is no such thing as chronic Lyme”.
The term chronic Lyme is also often applied to people who don’t feel well (fatigue, brain fog) but also haven’t ever had blood tests that show up positive for Borrelia burgdorferi. However, the testing for Borrelia is notoriously fickle with a high false negative rate. All of this murkiness leads to some doctors dismissing long-term Lyme symptoms.
Chronic Lyme as a polarizing topic leaves patients getting the short end of the stick. Encouragingly, research is now catching up on chronic Lyme symptoms and treatments, and more medical professionals are treating it seriously.
Researchers and doctors now use the term Post Treatment Lyme Disease (PTLD).
Let’s take a look at just some of the studies on PTLD showing the physiological and cellular changes in people with chronic Lyme.
What is Post Treatment Lyme Disease Syndrome (PTLD)?
After receiving standard treatment for Lyme (several weeks of antibiotics), some people continue to have ‘clinically relevant’ pain, fatigue, sleep disturbance, depression, and lower quality of life. Often, blood tests for these patients are normal. This has been well documented in several studies.[ref]
PTLD with significant symptoms affects between 10 and 20% of people treated for Lyme, according to official estimates. Other studies show that mild symptoms, whether ‘clinically relevant’ or not, remain for half or more of patients after treatment.[ref][ref]
Symptoms used in research studies to identify PTLD include:[ref][ref]
- muscle pain
- joint pain
- fatigue
- memory problems
- difficulties concentrating and problems finding words
- impact on daily activities
- sleep difficulties
- paresthesia
While these symptoms can also be due to other underlying causes, a recent study found that people with a history of Lyme disease were five times more likely to meet the criteria for PTLD than those with no prior Lyme diagnosis.[ref]
Long-term cognitive problems are common in chronic Lyme and a recent study shows that the symptoms continued without improvement for 12+ months after completing treatment. More encouragingly, the fatigue scores did improve a little over the course of a year.[ref]
What causes PTLD?
The question is: Why do 10 -20% of people treated for Lyme not recover completely?
Multiple possible root causes of PTLD are being investigated by researchers, and more than one could be going on at the same time.
- Bacteria (B.burgdorferi and others) remain in the body, even after aggressive antibiotics.[ref]
- The inability of some antibiotics to kill Borrelia sp. in biofilm-like structures.[ref]
- Inflammatory components of Borrelia sp. remain after the bacteria are killed.[ref]
- Multiple coinfections with undetermined pathogens.
- Cross-reactivity and mimicry of bacterial antigens to human tissues causing an autoimmune response.[ref]
With over 400,000 Lyme cases per year in the US alone, this is a growing population with chronic, debilitating symptoms. One study estimates that by 2020 the cumulative number of people dealing with PTLD could be as high as 1.9 million.[ref]
Multiple species:
While Lyme disease is traditionally linked with Borellia bacterial species (B. burgdorferi in the US), a study on biofilm disruption and antibiotic treatments found “multiple species of intracellular bacteria including rickettsia, Bartonella, Mycoplasma, Chlamydia, Tularemia, and Brucella contributing to the burden of illness and a high prevalence of Babesia complicating management with probable geographic spread of Babesia WA1/duncani to the Northeast.” The study also noted that a few individuals also had reactivation of various viral infections.[ref]
Lower initial immune response?
Research shows that patients with the bull’s-eye rash (erythema migrans) were 3-times less likely to end up with chronic Lyme when compared to patients with disseminated Lyme (later symptoms).[ref] The rash is indicative of a strong initial response to the bacteria and tick bite. Perhaps a stronger initial immune reaction decreases the risk of PTLD?
Bacterial pieces:
A recent study showed that non-viable components of B. burgdorferi stimulated a higher immune response in brain cells than the total bacteria.[ref] Components of the bacteria wouldn’t be affected by antibiotics, which may explain why taking more rounds of antibiotics doesn’t knock out the infection.
The peptidoglycan cell wall surrounding the B. burgdorferi bacteria is unique. The peptidoglycan is recognized by our immune system and causes an inflammatory response – but the unique B. burgdorferi peptidoglycan isn’t broken down and eliminated when the bacteria die. A study in mice showed that the peptidoglycan can linger in the liver and then end up stored in the joints. This triggers an inflammatory response in the joints, causing joint pain and arthritis symptoms seen in chronic Lyme.[ref — full study available, worth reading]
Metabolic differences:
A 2021 study looked at biomarkers in people with PTLD compared to a control group. The results showed that there were differences in glycerophospholipids, bile acid, and acetylcarnitine metabolites.[ref]
Microglial activation:
Overactive immune responses in the microglia of the brain may also be at the heart of PTLD for some. Research shows that antibiotic-killed spirochetes, such as Borrelia, can cause an increase in inflammatory proteins that is more robust than the live bacteria. The persistence of this inflammatory response in the central nervous system is theorized to be responsible for PTLD. Brain imaging shows that people with PTLD have activated microglia in the brain.[ref]
What is different in people with persistent Lyme?
Genetics can play a role in how well treatments for Lyme disease work and help us understand what is happening.
A 2019 study found that genetically based hyperinflammation may play a role. The chronic Lyme patients had imbalanced IL-6 along with elevated IL-1β and IL-8 (inflammatory cytokines).[ref]
Proteomics studies look at the differences in the blood proteins of people with chronic Lyme compared with people who had Lyme and recovered and those who never had Lyme. The research shows a clear difference in the blood proteins in people with PTLD.
A new study found that 35 biomarkers could be used to identify chronic Lyme. Several immune system pathways are involved. Interestingly, a couple of the genes are also related to epilepsy (CACNB4, ALDH7A1, SCN3A) which could be a molecular reason for the neurological symptoms of PTLD.[ref]
Chronic Lyme Genotype Report:
Lifehacks
If you find a tick and have symptoms of Lyme, research shows that the earlier you get treated, the better the odds are of a complete recovery.[ref][ref]
There are several ongoing clinical trials for different protocols to determine the best treatments for Lyme, so hopefully, more answers will be available soon.[ref]
Medication options:
Antibiotics:
Some research shows that more antibiotics after the initial course aren’t likely to help with Lyme.[ref][ref] Pulse dosing with ceftriaxone after other antibiotics doesn’t seem to work very well either.[ref]
A randomized controlled trial found that amoxicillin (20-day course) was a little more effective than azithromycin (7-day course).[ref] If you have a choice of antibiotics and no allergies to either, talk with your doctor about whether amoxicillin is a better choice.
A 2025 study found that a beta-lactam antibiotic called piperacillin can eliminate B. burgdorferi in vitro and in mice without harming the gut microbiome.[ref] Piperacillin is a broad-spectrum antibiotic that is FDA-approved and is used to treat hospital-acquired pneumonia and other serious infections.
Antibiotics plus dapsone for chronic Lyme:
Related Articles and Topics:
References:
Andreassen, Silje, et al. “Cognitive Function in Patients with Neuroborreliosis: A Prospective Cohort Study from the Acute Phase to 12 Months Post Treatment.” Brain and Behavior, vol. 12, no. 6, June 2022, p. e2608. PubMed, https://doi.org/10.1002/brb3.2608.
Aucott, John N., et al. “Risk of Post-Treatment Lyme Disease in Patients with Ideally-Treated Early Lyme Disease: A Prospective Cohort Study.” International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases, vol. 116, Mar. 2022, pp. 230–37. PubMed, https://doi.org/10.1016/j.ijid.2022.01.033.
Berende, Anneleen, Hadewych J. M. Ter Hofstede, et al. “Effect of Prolonged Antibiotic Treatment on Cognition in Patients with Lyme Borreliosis.” Neurology, vol. 92, no. 13, Mar. 2019, pp. e1447–55. PubMed, https://doi.org/10.1212/WNL.0000000000007186.
Berende, Anneleen, Hadewych J. M. ter Hofstede, et al. “Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease.” The New England Journal of Medicine, vol. 374, no. 13, Mar. 2016, pp. 1209–20. PubMed, https://doi.org/10.1056/NEJMoa1505425.
Bockenstedt, Linda K., et al. “Spirochete Antigens Persist near Cartilage after Murine Lyme Borreliosis Therapy.” The Journal of Clinical Investigation, vol. 122, no. 7, July 2012, pp. 2652–60. PubMed Central, https://doi.org/10.1172/JCI58813.
CDC. “Concern about Lyme Disease | CDC.” Centers for Disease Control and Prevention, 13 Jan. 2021, https://www.cdc.gov/lyme/why-is-cdc-concerned-about-lyme-disease.html.
—. “How Many People Get Lyme Disease? | CDC.” Centers for Disease Control and Prevention, 13 Jan. 2021, https://www.cdc.gov/lyme/stats/humancases.html.
—. “Lyme Disease Home | CDC.” Centers for Disease Control and Prevention, 19 Jan. 2022, https://www.cdc.gov/lyme/index.html.
Chaconas, George, et al. “Changing of the Guard: How the Lyme Disease Spirochete Subverts the Host Immune Response.” The Journal of Biological Chemistry, vol. 295, no. 2, Jan. 2020, pp. 301–13. PubMed, https://doi.org/10.1074/jbc.REV119.008583.
Clarke, Daniel J. B., et al. “Gene Set Predictor for Post-Treatment Lyme Disease.” Cell Reports Medicine, vol. 3, no. 11, Nov. 2022. www.cell.com, https://doi.org/10.1016/j.xcrm.2022.100816.
Coburn, Jenifer, et al. “Lyme Disease Pathogenesis.” Current Issues in Molecular Biology, vol. 42, 2021, pp. 473–518. PubMed Central, https://doi.org/10.21775/cimb.042.473.
Custodio, Marco M., et al. “Disulfiram: A Repurposed Drug in Preclinical and Clinical Development for the Treatment of Infectious Diseases.” Anti-Infective Agents, vol. 20, no. 3, June 2022, p. e040122199856. PubMed, https://doi.org/10.2174/2211352520666220104104747.
DeLong, Allison, et al. “Estimation of Cumulative Number of Post-Treatment Lyme Disease Cases in the US, 2016 and 2020.” BMC Public Health, vol. 19, no. 1, Apr. 2019, p. 352. PubMed, https://doi.org/10.1186/s12889-019-6681-9.
Eliassen, Knut Eirik, et al. “Symptom Load and General Function among Patients with Erythema Migrans: A Prospective Study with a 1-Year Follow-up after Antibiotic Treatment in Norwegian General Practice.” Scandinavian Journal of Primary Health Care, vol. 35, no. 1, Mar. 2017, pp. 75–83. PubMed, https://doi.org/10.1080/02813432.2017.1288812.
Embers, Monica E., et al. “Persistence of Borrelia Burgdorferi in Rhesus Macaques Following Antibiotic Treatment of Disseminated Infection.” PLOS ONE, vol. 7, no. 1, Jan. 2012, p. e29914. PLoS Journals, https://doi.org/10.1371/journal.pone.0029914.
Estrada-Peña, Agustín, et al. “An Updated Meta-Analysis of the Distribution and Prevalence of Borrelia Burgdorferi s.l. in Ticks in Europe.” International Journal of Health Geographics, vol. 17, no. 1, Dec. 2018, p. 41. BioMed Central, https://doi.org/10.1186/s12942-018-0163-7.
“Factsheet about Borreliosis.” European Centre for Disease Prevention and Control, https://www.ecdc.europa.eu/en/borreliosis/facts/factsheet. Accessed 17 Nov. 2022.
Feng, Jie, Shuo Zhang, et al. “Ceftriaxone Pulse Dosing Fails to Eradicate Biofilm-Like Microcolony B. Burgdorferi Persisters Which Are Sterilized by Daptomycin/ Doxycycline/Cefuroxime without Pulse Dosing.” Frontiers in Microbiology, vol. 7, 2016, p. 1744. PubMed, https://doi.org/10.3389/fmicb.2016.01744.
Feng, Jie, Megan Weitner, et al. “Eradication of Biofilm-Like Microcolony Structures of Borrelia Burgdorferi by Daunomycin and Daptomycin but Not Mitomycin C in Combination with Doxycycline and Cefuroxime.” Frontiers in Microbiology, vol. 7, 2016, p. 62. PubMed, https://doi.org/10.3389/fmicb.2016.00062.
Geebelen, Laurence, et al. “Non-Specific Symptoms and Post-Treatment Lyme Disease Syndrome in Patients with Lyme Borreliosis: A Prospective Cohort Study in Belgium (2016-2020).” BMC Infectious Diseases, vol. 22, no. 1, Sept. 2022, p. 756. PubMed, https://doi.org/10.1186/s12879-022-07686-8.
Goc, A., A. Niedzwiecki, et al. “In Vitro Evaluation of Antibacterial Activity of Phytochemicals and Micronutrients against Borrelia Burgdorferi and Borrelia Garinii.” Journal of Applied Microbiology, vol. 119, no. 6, Dec. 2015, pp. 1561–72. PubMed Central, https://doi.org/10.1111/jam.12970.
—. “Reciprocal Cooperation of Phytochemicals and Micronutrients against Typical and Atypical Forms of Borrelia Sp.” Journal of Applied Microbiology, vol. 123, no. 3, Sept. 2017, pp. 637–50. PubMed, https://doi.org/10.1111/jam.13523.
Goc, Anna, Aleksandra Niedzwiecki, et al. “Anti-Borreliae Efficacy of Selected Organic Oils and Fatty Acids.” BMC Complementary and Alternative Medicine, vol. 19, no. 1, Feb. 2019, p. 40. PubMed, https://doi.org/10.1186/s12906-019-2450-7.
Goc, Anna, Alexandra Niedzwiecki, et al. “Cooperation of Doxycycline with Phytochemicals and Micronutrients Against Active and Persistent Forms of Borrelia Sp.” International Journal of Biological Sciences, vol. 12, no. 9, July 2016, pp. 1093–103. PubMed Central, https://doi.org/10.7150/ijbs.16060.
Hammer, Christian, et al. “A Coding Variant of ANO10, Affecting Volume Regulation of Macrophages, Is Associated with Borrelia Seropositivity.” Molecular Medicine, vol. 21, no. 1, Jan. 2015, pp. 26–37. Springer Link, https://doi.org/10.2119/molmed.2014.00219.
Hein, Tabea M., et al. “Cytokine Expression Patterns and Single Nucleotide Polymorphisms (SNPs) in Patients with Chronic Borreliosis.” Antibiotics, vol. 8, no. 3, July 2019, p. 107. PubMed Central, https://doi.org/10.3390/antibiotics8030107.
Jowett, Nate, et al. “Steroid Use in Lyme Disease-Associated Facial Palsy Is Associated with Worse Long-Term Outcomes: Steroids in Lyme Disease Facial Palsy.” The Laryngoscope, vol. 127, no. 6, June 2017, pp. 1451–58. DOI.org (Crossref), https://doi.org/10.1002/lary.26273.
Liegner, Kenneth B. “Disulfiram (Tetraethylthiuram Disulfide) in the Treatment of Lyme Disease and Babesiosis: Report of Experience in Three Cases.” Antibiotics, vol. 8, no. 2, June 2019, p. 72. www.mdpi.com, https://doi.org/10.3390/antibiotics8020072.
Ljøstad, U., and A. Mygland. “Remaining Complaints 1 Year after Treatment for Acute Lyme Neuroborreliosis; Frequency, Pattern and Risk Factors.” European Journal of Neurology, vol. 17, no. 1, Jan. 2010, pp. 118–23. PubMed, https://doi.org/10.1111/j.1468-1331.2009.02756.x.
“Lyme Disease: What’s Actually Going on Here?” Pharmaceutical Technology, 2 Apr. 2020, https://www.pharmaceutical-technology.com/analysis/lyme-disease-whats-actually-going-on-here/.
Lyon, Joanna, and Hyunuk Seung. “Genetic Variation in the ABCB1 Gene Associated with Post Treatment Lyme Disease Syndrome Status.” Meta Gene, vol. 21, Sept. 2019, p. 100589. ScienceDirect, https://doi.org/10.1016/j.mgene.2019.100589.
Murray, Lilly, et al. “Kundalini Yoga for Post-Treatment Lyme Disease: A Preliminary Randomized Study.” Healthcare (Basel, Switzerland), vol. 10, no. 7, July 2022, p. 1314. PubMed, https://doi.org/10.3390/healthcare10071314.
Parthasarathy, Geetha, and Shiva Kumar Goud Gadila. “Neuropathogenicity of Non-Viable Borrelia Burgdorferi Ex Vivo.” Scientific Reports, vol. 12, no. 1, Jan. 2022, p. 688. PubMed, https://doi.org/10.1038/s41598-021-03837-0.
Radolf, Justin D., et al. “Lyme Disease in Humans.” Current Issues in Molecular Biology, vol. 42, 2021, pp. 333–84. PubMed Central, https://doi.org/10.21775/cimb.042.333.
Rebman, Alison W., et al. “The Clinical, Symptom, and Quality-of-Life Characterization of a Well-Defined Group of Patients with Posttreatment Lyme Disease Syndrome.” Frontiers in Medicine, vol. 4, 2017. Frontiers, https://www.frontiersin.org/articles/10.3389/fmed.2017.00224.
Schröder, Nicolas W. J., et al. “Heterozygous Arg753Gln Polymorphism of Human TLR-2 Impairs Immune Activation by Borrelia Burgdorferi and Protects from Late Stage Lyme Disease.” Journal of Immunology (Baltimore, Md.: 1950), vol. 175, no. 4, Aug. 2005, pp. 2534–40. PubMed, https://doi.org/10.4049/jimmunol.175.4.2534.
Steere, Allen C., et al. “Antibiotic-Refractory Lyme Arthritis Is Associated with HLA-DR Molecules That Bind a Borrelia Burgdorferi Peptide.” The Journal of Experimental Medicine, vol. 203, no. 4, Apr. 2006, pp. 961–71. PubMed Central, https://doi.org/10.1084/jem.20052471.
Strle, Klemen, et al. “A Toll-like Receptor 1 Polymorphism Is Associated with Heightened T-Helper 1 Inflammatory Responses and Antibiotic-Refractory Lyme Arthritis.” Arthritis and Rheumatism, vol. 64, no. 5, May 2012, pp. 1497–507. PubMed Central, https://doi.org/10.1002/art.34383.
Vrijmoeth, Hedwig D., et al. “Prevalence and Determinants of Persistent Symptoms after Treatment for Lyme Borreliosis: Study Protocol for an Observational, Prospective Cohort Study (LymeProspect).” BMC Infectious Diseases, vol. 19, no. 1, Apr. 2019, p. 324. PubMed, https://doi.org/10.1186/s12879-019-3949-8.