Key takeaways:
~ Reelin is a glycoprotein that is essential for brain development in babies and children and cognitive function in adults.
~ Reelin interacts with receptors in the brain (ApoER2, VLDLR, and NMDA). Altered reelin levels are found in individuals with Alzheimer’s, schizophrenia, autism, and depression.
~ Genetic and epigenetic changes can increase the risk of schizophrenia, autism, major depressive disorder, and Alzheimer’s.
What is Reelin?
Reelin is a secreted glycoprotein with multiple functions in the body, including prenatal development and adult brain function. Reelin was discovered from a mutation in the RELN gene that caused mice to have a reeling gate.[ref]
- During prenatal development, it is important in neuronal migration and brain development.[ref]
- In adults, reelin is involved in synaptic plasticity, brain function, memory, cognition, and the creation of new brain cells. It keeps the adult brain dynamic and resilient.
Studies on reelin and genetic variants that alter the RELN gene show that it plays a role in schizophrenia, bipolar disorder, psychosis, Alzheimer’s disease, and even autism. Decreased reelin levels are found in many neuropsychiatric and neurodegenerative disorders.[ref][ref]
Reelin also plays important roles outside the brain. In cardiovascular disease and non-neuronal inflammation, reducing high reelin levels may be beneficial.[ref]
Thus, lower levels of reelin may help with cardiovascular disease, while higher levels of reelin may prevent or reverse neurodegenerative disease.
In the intestines, reelin is also found in all intestinal layers in the enteric neurons and plays a role in how the intestinal cells turn over and renew.[ref][ref]
Keep in mind throughout the discussions of higher or lower reelin levels that the measurements are usually plasma reelin that is circulating in the bloodstream. There are still many unknowns about reelin levels in the brain.
Let’s take a look at how reelin is made and the receptors that bind to it. Then I’ll explain the roles of higher or lower reelin in neurological conditions, long covid, and heart disease. We will also look at the genetic variants in the RELN gene, and finish with the ways in which reelin can be upregulated or downregulated.
Reelin synthesis and secretion:
The RELN gene codes for the reelin protein. It is synthesized as a large protein that then undergoes glycosylation, a process in which sugar molecules are attached to the protein.
Reelin is then cleaved into smaller proteins that activate specific receptors.
Certain types of neurons secrete reelin in the brain. It primarily interacts with glutamatergic and GABAergic neurons. Glutamate is an excitatory neurotransmitter, while GABA is an inhibitory neurotransmitter, and balance is needed for a healthy brain.
Receptors for reelin: APOER2 and VLDLR
Like many proteins, reelin can bind to a receptor to cause an action to take place.
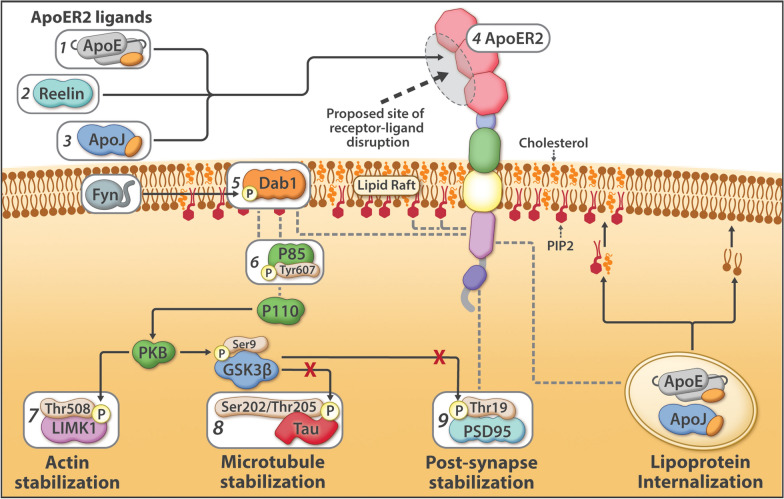
There are two main receptors that reelin binds to: apolipoprotein E receptor 2 (ApoER2, encoded by LRP8 gene) and very low-density lipoprotein receptor (VLDLR).[ref]
When reelin binds to the receptors, it activates a protein in cells called DAB1, which is important in neuronal development and brain health.
ApoER2:
The LRP8 gene encodes the apolipoprotein E receptor. This receptor is found in the central nervous system, brain, olfactory bulb, placenta, and testes. In addition to binding with reelin, ApoER2 can bind to apolipoprotein E (ApoE), which is a cholesterol transporter in the brain. Reelin can also bind to thrombospondins, which are extracellular matrix proteins involved in neuronal migration and synapse formation. Finally, ApoER2 can also bind to selenoprotein P, resulting in the transport of selenium into the cell. [ref]
VLDLR:
The very low-density lipoprotein receptor is similar to ApoER2. In addition to binding to reelin, VLDLR also binds to thrombospondins and ApoE. However, it doesn’t bind selenoprotein P. [ref]
Reelin also interacts with the NMDA receptor through connections with the ApoE receptor. The NMDA receptor is involved in neurotransmission from glutamate. Reelin regulates a subunit of the NMDA receptor and in this way affects neuronal plasticity. Studies show that Reelin enhances the glutamate signal through the NMDA receptor.[ref][ref][ref]
Related article: Glutamate balance and receptors
DAB1: Signaling pathway
Reelin binds to the ApoER2 and VLDLR receptors which then interact with DAB1 inside the cell, causing it to bind to other proteins and activate a signaling cascade.
- During brain development, the Reelin-Dab1 pathway activation is what regulates neurons moving into the right position in the brain.
- In the adult brain, the same pathway is involved in neuronal function and synaptic plasticity.
The ApoER2-DAB1 pathway is important in Alzheimer’s disease.
Alzheimer’s Disease (AD): Integral associations with Reelin, Dab1, and ApoER2
Reelin mutation prevents early-onset AD: Alzheimer’s disease can be divided into two categories: early-onset disease caused by rare mutations (about 1-2% of Alzheimer’s cases) or late-onset disease which is a combination of environmental factors plus genetic susceptibility. Early onset Alzheimer’s can occur around age 50 and is caused by inheriting a rare mutation in the APP (amyloid precursor protein), PSEN1 (presenilin 1), or PSEN2 (presenilin 2). The rare mutations cause early-onset Alzheimer’s nearly 100% of the time, with some variations as to the age of onset.
In 2023, researchers found that a mutation in RELN (reelin gene) prevented early onset Alzheimer’s in someone with a PSEN1 mutation. The person in the study should have gotten early-onset Alzheimer’s around age 50, based on the PSEN1 mutation, but remained cognitively fine until around age 70. After he passed away, an autopsy showed that his brain was filled with amyloid plaque, but that the tau tangles that are also involved in Alzheimer’s weren’t present in the part of the brain that involves memory. The researchers found that he had a rare mutation in the RELN gene. The researchers then created a mouse model with the mutation, showing reduced tau tangles.[ref]
The RELN mutation that protects against Alzheimer’s is a gain-of-function mutation that increases reelin in the brain. This is a very strong indicator that higher Reelin levels may help to protect against Alzheimer’s.
While early-onset, familial Alzheimer’s is rare, research also shows that increased Reelin or increased Reelin-Dab1 activation is associated with a lower risk of Alzheimer’s disease.
Studies on Alzheimer’s brain show that there is an increase in RELN mRNA in the frontal cortex of the brain in advanced AD, however, the reelin protein levels were not increased (mRNA not translated into the reelin protein). I mentioned above that the reelin protein is a large glycoprotein that is cleaved into several smaller proteins. Even with increased RELN mRNA, Alzheimer’s brains show lower levels of the full-length Reelin protein and one specific length of cleaved protein. It could be that the different cleavage protein lengths are playing a role in Alzheimer’s.[ref]
Excess glutamate in the synapse may also play a role in Alzheimer’s pathology. Amyloid-beta plaque interferes with the availability of the NMDA glutamate receptors, which is thought to play a role in neuronal loss. Reelin can counter this through the interaction with ApoeR2 and the NMDA receptor. Researchers think that: “at high concentrations of Aβ peptides, Reelin can no longer overcome the Aβ induced functional suppression” of the NMDA receptor.[ref]
Neurofibril tau tangles: Disruption of the ApoER2-DAB1 pathway has recently been discovered by NIH researchers to be at the core of the Tau neurofibrillary tangles that accumulate in the brain in Alzheimer’s patients. Part of the DAB1 signaling pathway prevents Tau phosphorylation, essentially preventing the beginning of the Tau tangle. Disruption then allows for the Tau tangle to form. Disruption of the ApoER2-DAB1 pathway also affects synapse strength and the delivery of cholesterol to the neuron.[ref]
Interaction with APOE E4: The largest genetic risk factor for late-onset Alzheimer’s is the APOE E4 allele. Research shows that the APOE E4 allele impairs synaptic plasticity by reducing the recycling of the ApoER2 receptor to the cell surface.
Along the same line, the E4 allele reduces the NMDA and AMPA (glutamate) receptors on the cell membrane. Without sufficient ApoER2 receptors, the positive effects of Reelin on the neurons are missing. Combined with the reduced effect on the glutamate receptors, the decreased Reelin receptor binding is part of what drives neuronal degeneration in Alzheimer’s.[ref]
A study in people with two copies of the APOE E4 allele (high risk of Alzheimer’s) showed that a DAB1 genetic variant reduced the risk of getting Alzheimer’s.[ref] This indicates that increasing DAB1 may also be a way of mitigating Alzheimer’s risk.
Related article: How to determine your APOE type (if you want to know)
Reelin, Dab1, and ApoER2 levels are a hot area of research right now for AD, and I’m hopeful that more answers are soon to come.
Schizophrenia:
Schizophrenia is a serious mental health condition that usually begins in early adulthood. People with schizophrenia may have hallucinations, delusions, and disorganized thinking.
In patients with schizophrenia, RELN mRNA and protein levels are lower than normal. Similarly, in post-mortem brain samples RELN mRNA is decreased by 30-50% in schizophrenia.[ref][ref]
What changes the RELN levels?
- Studies show that some people with schizophrenia have increased RELN methylation leading to a 25-fold decrease in reelin levels.[ref][ref]
- Genetic variants in the RELN gene alter expression and increase the relative risk of schizophrenia.
Thus, in schizophrenia, there can either be a genetic or epigenetic reduction in reelin levels in the brain, which affects brain development and changes synaptic plasticity in the adult brain.
Reelin in long Covid:
Long Covid perturbs homeostasis in ways that involve multiple pathways, multiple systems — unique to an individual and changing over time.
- For some, long Covid symptoms could be due to endothelial dysfunction (increased reelin) and the persistence of the virus in the endothelium or intestines.
- Others have symptoms that point towards a neurological cause including brain fog and severely diminished energy (possibly low reelin in the brain).
A recent study found that reelin is “notably elevated” in the plasma of long Covid patients. The study looked at the mRNA levels in long Covid patients (n=47) and in a matched, healthy cohort. Looking at a broad picture of mRNA levels shows which genes are upregulated or downregulated in long Covid. The results showed several neuro/inflammation genes were upregulated, but reelin stood out as one of the top upregulated genes.[ref] What is unknown here is whether reelin was continually upregulated due to viral persistence — and what the reelin levels in the brain were, in comparison to the plasma levels.
Higher reelin levels can interact in the endothelium of the blood vessels to increase inflammation, activate platelets, and increase clotting (more below). Reelin levels are increased during severe Covid infections and are thought to play a role in hyperinflammation and increased clotting.[ref] Another study found that reelin levels were elevated along with inflammatory markers (IL-1α, IL-4) during Covid disease progression, but that plasma reelin levels returned to normal in long Covid.[ref][ref]
Keep in mind that reelin modulates NMDA receptor activity through interaction with Dab1, and glutamate regulation is also implicated in long Covid.
Related article: Glutamate receptors and regulation
Interestingly, a study in Covid patients found anti-neuronal antibodies that are predicted to bind to Dab1. The researchers looked at brainstem-related proteins and found three that shared potentially immunogenic sequences similar to proteins in SARS-CoV-2. They tested the blood of 23 severely affected Covid patients and found that 14 of them had antibodies that would bind not only to the virus but also to DAB1.[ref]
It would be interesting to see a lot more research on foundational neuronal proteins like Dab1 and reelin in regard to long Covid.
Autism:
Reelin has been extensively investigated for its role in autism since it is integral to synaptic formation and neuronal migration. Children with autism have excess neuronal growth in certain areas of the brain, but this changes by adulthood.[ref]
RELN mRNA is reduced in the brains of autism patients (postmortem). Reelin levels are also low in the plasma and brains of adults with autism.[ref] Epigenetic studies show that RELN mRNA levels are decreased in autism due to DNA methylation.[ref]
However, reelin is likely not acting alone in autism. Some children with autism have normal Reelin levels, while in one study, half of the children with autism had up to 30-fold higher than normal reelin levels.[ref]
Genetic studies also point to the role of reelin in autism spectrum disorder. Variants in the RELN gene are associated with an increased relative risk of autism.[ref] Other studies show that a combined haplotype of variants in RELN and DAB1 increases the risk for autism.[ref]
Depression:
Neuronal inflammation is one cause of depression, and studies show that reelin expression in the brain can be downregulated (lower levels) in major depressive disorder.[ref][ref]
A recent study showed that people have a decrease in reelin during a depression episode and that reelin levels return to normal after depression is treated and in remission.[ref]
In animals, intervenous Reelin injections reverse despair-like behavior. It also normalizes behavior in an animal model of post-partum depression. [ref][ref]
Keep in mind that reelin and neuroinflammation aren’t the only causes of depression, but it is an interesting connection for some with major depressive disorder.
Related article: Genetic pathways and depression
Peripheral Reelin Levels:
While much of the research on reelin involves schizophrenia, neurodegenerative diseases, and cognitive function, this glycoprotein signal is important outside of the brain as well.
Let’s switch gears and look at other ways that reelin is important in the peripheral nervous system and in the vascular endothelium.
Reelin in cardiovascular disease and chronic inflammatory diseases:
Outside of the brain, reelin is also abundant in the blood and interacts with the endothelial cells lining the blood vessels. In atherosclerosis (plaque in the arteries), higher Reelin levels increase inflammatory mediators. Reelin also stimulates platelet adhesion (increased clotting risk) and decreases endothelial nitric oxide. All of these actions are a negative when it comes to heart health.[ref]
Plasma Reelin depletion is being tested for reducing heart disease. Researchers have looked at ways to reduce Reelin for decades using monoclonal antibodies as well as other ways in lab animals. The problem is that reducing Reelin in ways that affect levels in the brain causes significant neurological issues.
Otosclerosis and hearing loss:
Several genetic studies have repeatedly found that RELN genetic variants alter the statistical risk of otosclerosis, which causes hearing loss by ossification of the stapes bone in the ear. While this was initially puzzling to researchers, a recent study does show that RELN is expressed in the stapes tissues and that reduced RELN expression is found in otosclerosis.[ref]
Nerve regeneration:
Reelin also plays a role in peripheral nerve regeneration after an injury. Animal studies show reelin is secreted by Schwann cells in peripheral nerves following injury, and that a lack of reelin impairs nerve regeneration.[ref]
Interestingly, progesterone stimulates the expression of Reelin in peripheral nerves in animals.[ref]
Reelin: Gene Expression and Rare Mutations
Mutations in the reelin gene can have significant effects on brain development, as researchers first noticed with the strain of mice that had a reeling gate and other disability. In addition to genetic variants (in the genotype report below) and rare mutations, the level of RELN expression is controlled by multiple gene expression mechanisms.
Lissencephaly:
Rare loss-of-function mutations in the RELN gene can cause lissencephaly, which is a condition known as “smooth brain”. Essentially, the mutations and loss of reelin cause the brain to form without the normal folds in it. It can cause intellectual and physical disability as well as seizures. In individuals with only one copy of a loss-of-function mutation, they will have changes to the cortical structure but not the cerebellum.[ref]
Regulating RELN Gene Expression:
MicroRNAs (miRNAs) are short strands of RNA that can bind to mRNA and control whether the mRNA is translated into its protein.
Increased levels of MiR-128 cause a decrease in reelin in cell line studies.[ref] MiR-128 is upregulated in Alzheimer’s disease and autism.[ref] On the other hand, increased miR-128 is beneficial in the blood vessels.[ref]
A preprint study shows that MiR-384 regulates ADAMTS4, which is the protease that cleaves the large reelin protein into its fragments that bind to the receptors. Overexpression of miR-384 causes lower ADAMTS4 levels which then leads to lower levels of cleaved reelin and lower receptor activation.[ref]
EGR3 is a transcription factor that regulates reelin expression. EGR3 has also been implicated in schizophrenia.[ref]
My point here is that there are multiple ways that Reelin expression is controlled in the brain and in the periphery. All of these gene expression pathways target multiple genes and affect the expression of genes in different pathways — adding a layer of complexity here.
Supplemental Reelin in the Brain:
What happens when you increase reelin in the brain?
Animal studies show that increasing reelin, either by injection into the brain or by peripheral injection, has positive effects on memory and spatial learning.[ref] Injecting reelin also reverses the cognitive deficits in a mouse model for Angelman syndrome. Angleman syndrome is a very rare genetic disorder that causes developmental delays, movement problems, and intellectual disability.[ref]
Genotype report: RELN and DAB1
Access this content:
An active subscription is required to access this content.
Lifehacks:
In mice, injecting reelin into the brain enhances memory, learning, and performance in models of neurodegeneration. However, a needle into the brain isn’t a readily available option in humans.
Reelin is a large protein that was initially thought to not be able to cross the blood-brain barrier. More recently, studies point to the possibility of a transporter to move reelin into the brain. Peripheral doses of reelin have a rapid anti-depressant effect, which makes researchers think it must be getting to the brain.[ref]
Keep in mind that increasing reelin may only be beneficial if reelin levels are low, such as in neurodegenerative diseases or some cases of depression. High reelin levels in heart disease may be a negative in regard to platelet stickiness.
Environmental factors that decrease reelin:
Organophosphates:
Chlorpyrifos is a commonly used organophosphate pesticide. Prenatal exposure in animals causes changes in behavior similar to autism. Similarly, low reelin levels cause the same changes in behavior. However, researchers found that the combination of chlorpyrifos with low reelin had a paradoxical effect and prevented the ASD-like behavior.[ref] In adult animals, chlorpyrifos exposure along with low reelin causes autism-like behavior.[ref]
Glyphosate-based herbicide exposure:
Studies in animals show that glyphosate-base herbicides affect brain development in offspring through affecting how neuronal cells migrate to the right location. In adult animals, glyphosate-based herbicides are associated with an upregulation of Reelin and Dab1, which indicates a compensatory mechanism in response to the herbicide.[ref]
Supplements that upregulate reelin:
Access this content:
An active subscription is required to access this content.
Related articles and topics:
Neuropilins: Neurodevelopmental Disorders, Gut Barrier, & Covid Brain