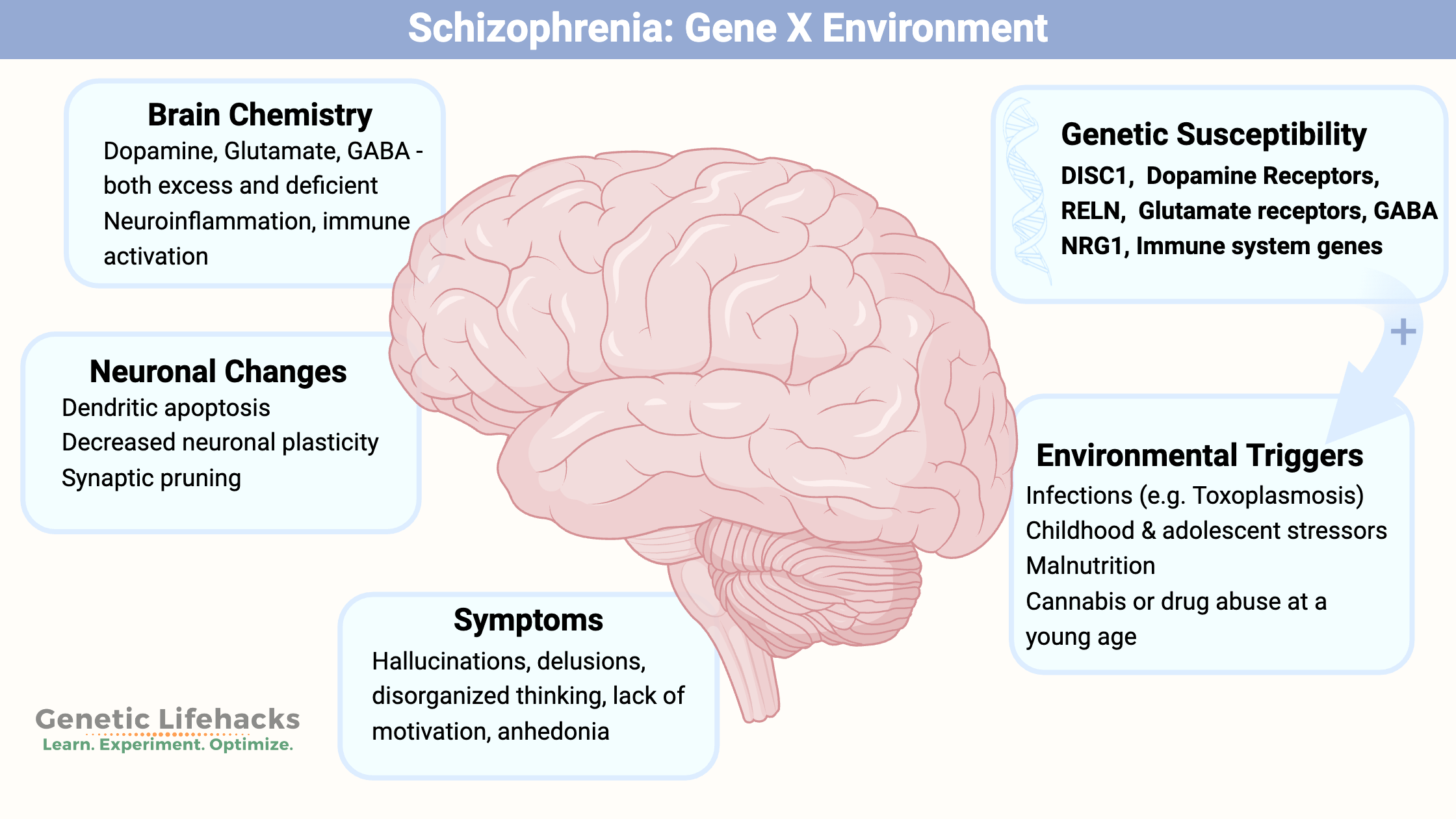
Key takeaways:
~ Schizophrenia involves multiple pathways in the brain.
~ Genetic variants play a large role in susceptibility to schizophrenia, but genetics alone doesn’t cause schizophrenia for most.
~ Immune system challenges and other stressors at specific times of brain development are also involved.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.
Schizophrenia: Genes, brain changes, and pathways involved
Schizophrenia is a complex psychiatric disorder affecting about 1% of the population. It typically starts between ages 15-35 in men and late 20s to early 30s in women. Symptoms include disorganized thinking, lack of motivation, anhedonia, psychosis, delusions, hallucinations, and disorganized speech.[ref][ref][ref]
Twin studies estimate the heritability of schizophrenia at ~80%, indicating a strong genetic component. This means that genetic variants play a large role in susceptibility, but it isn’t genes alone that cause schizophrenia. Instead, genetic susceptibility combined with environmental factors, such as immune system activation or childhood neglect, come together in the brain changes seen in schizophrenia.[ref][ref]
This article will cover:
- Brain chemistry changes in schizophrenia
- Current genetic research on associated variants and pathways
- Environmental factors involved in schizophrenia development
Brain Chemistry Changes in Schizophrenia
Neurons in the brain communicate by releasing neurotransmitters, which are then taken up by nearby neurons. While the simplified pictures show a single neurotransmitter released and then received, in reality, many neurons release multiple neurotransmitters and have various receptor types. Keep this complexity in mind as we discuss dopamine, glutamate, GABA, and other neurotransmitter systems in schizophrenia.
Dopamine imbalances:
Dopamine is a neurotransmitter that plays key roles in movement, motivation, and learning.
The dopamine hypothesis of schizophrenia comes from initial studies in the 1970s that showed symptom reduction when the dopamine receptor D2 (DRD2) was blocked using antipsychotic drugs. Dopamine acts by binding to five different receptors (D1 – D5). Excess dopamine was thought to be the cause of hallucinations (e.g. hearing voices) and delusions.
Recent research reveals a more nuanced picture, with dopamine levels higher in some brain regions and lower in others.[ref] This imbalance may explain various symptoms: higher dopamine in certain areas might cause hallucinations and paranoia, while lower dopamine receptor activation elsewhere could relate to a lack of motivation and anhedonia.[ref]
Related article: Dopamine synthesis | Dopamine receptors
Glutamate abnormalities:
Glutamate is the most abundant excitatory neurotransmitter and affects memory, learning, and mood. It binds to several different receptors, including the NMDA receptor. Drugs that are NMDA antagonists, like ketamine, can imitate some symptoms of schizophrenia. Glutamate is involved in attention, perception, brain plasticity, and brain development through adolescence.[ref]
Related articles: Glutamate transport and receptors | Glutamate synthesis
GABA:
GABA is the main inhibitory neurotransmitter – essentially turning off the action of the excitatory neurotransmitters. Like glutamate and dopamine, GABA also acts by binding with several different receptors.
Insufficient GABA (not enough of the “off signal”) can lead to an increase in dopamine and a dysregulation of NMDA receptors. Research shows that when GABA is decreased in the frontal cortex, emotional, behavioral, and cognitive changes occur.[ref] GABA is synthesized from glutamate using the GAD67 enzyme, and postmortem studies show reduced GAD67 in the prefrontal cortex in schizophrenia patients.[ref]
Related article: GABA synthesis and receptors
Serotonin:
Serotonin’s role in schizophrenia was proposed after Albert Hoffman’s discovery that LSD intensifies serotonin effects. Brain imaging studies show hyperactivity in serotonergic pathways in schizophrenia. It is now thought that serotonin may play a role only in a subset of schizophrenia patients.[ref][ref]
Related article: Serotonin genes
Altered Brain Development and Function:
Beyond neurotransmitter imbalances, schizophrenia involves alterations in brain development, structure, and function:
Brain development and synaptic pruning:
The final phase of brain development happens during adolescence with changes in brain structure, decreasing grey matter, and increasing white matter volumes. In all mammals, there is an overproduction of neurons, synapses, and axons during development which are then pruned during maturation. Neuronal synapses and dendrites are pruned and reorganized in brain maturation during adolescence. The initial pruning happens via the complement system – part of the innate immune system. The complement system proteins are active in the brain at low levels under normal conditions, but complement factors can be upregulated when a pathogen is in the brain. Animal models show that overexpression of complement factor C4 causes excessive prefrontal cortex synaptic pruning and symptoms similar to schizophrenia.[ref] We will circle back to complement system activation in both the genetic susceptibility and environmental factors.
Default Mode Network (DMN):
The Default Mode Network (DMN) is a network of brain regions that are active when we are not focused on the outside world. It is deactivated when you start a task, but the DMN kicks in when you aren’t thinking about doing something — like driving a car down the highway when there isn’t any traffic. In people with schizophrenia, the DMN may not function properly, leading to difficulties with self-awareness, hallucinations, and delusions. Brain imaging studies show that in people with schizophrenia, there is abnormal activity in the DMN with different tasks.[ref]
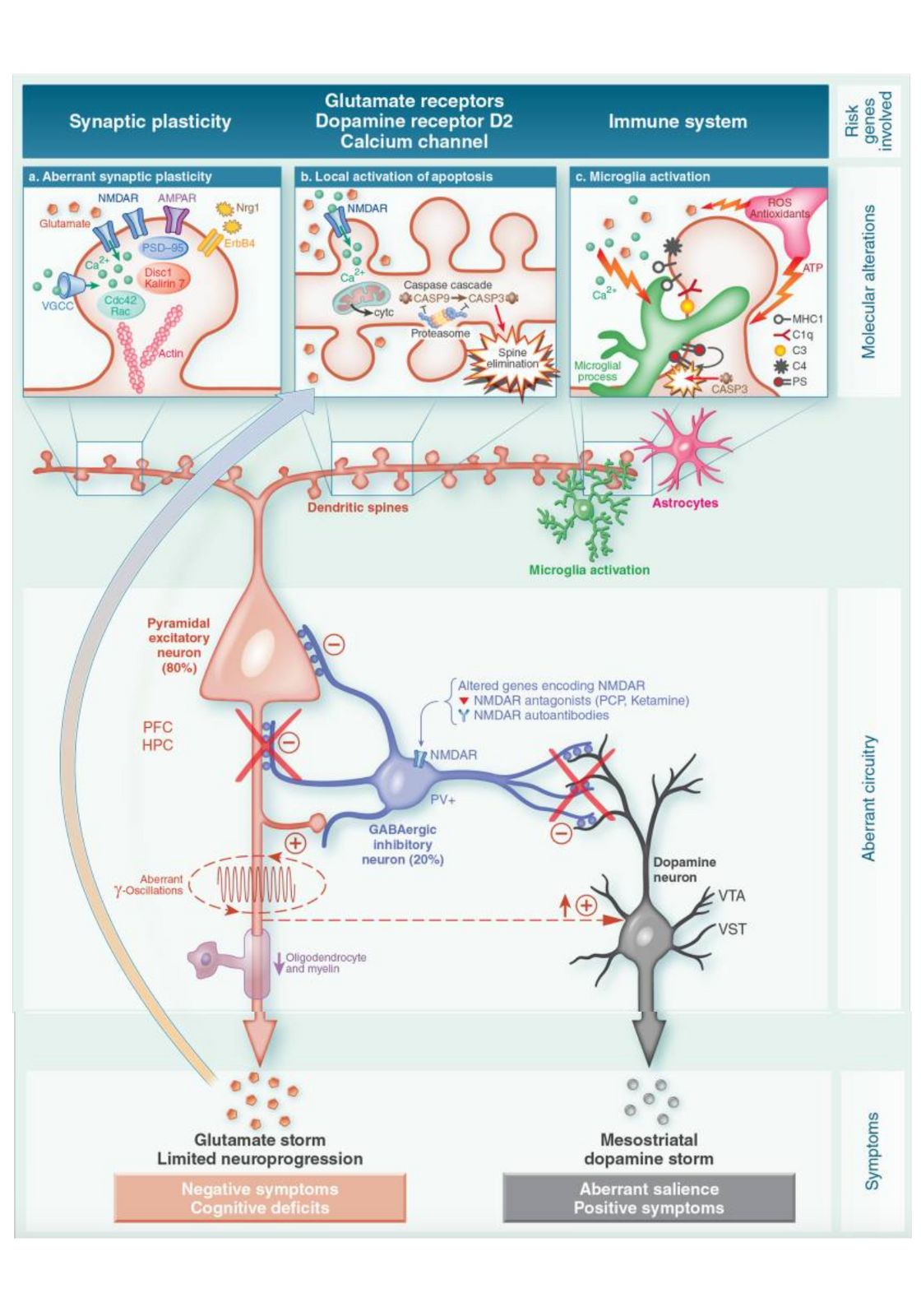
Tying together the altered brain chemistry and physiological changes:
The image below (from this paper) shows the interactions between neurotransmitters (glutamate, dopamine, GABA) and the brain’s immune system in altering the function of neurons in schizophrenia:
Causes of Schizophrenia: Why does it happen?
So far we’ve touched on the physical and physiological changes in the schizophrenic brain, but this doesn’t answer the question of “why?”
Schizophrenia typically emerges during the final phase of brain development (late teens to young adulthood). The two-hit or multi-hit model proposes that genetic susceptibility combined with life events or environmental ‘hits’ leads to schizophrenia. Environmental ‘hits’ may include prenatal or early childhood brain development stressors (e.g., maternal stress, delivery complications, malnutrition, immune system challenges) and later factors like childhood trauma, neglect, sexual abuse, parental loss, or early drug use.[ref]
Let’s take a look at the genetic risk and then specific environmental factors.
Genetic susceptibility to schizophrenia:
Another way to investigate the cause of a condition is to look at the genetic variants that increase or decrease susceptibility to see which pathways are involved.
Researchers have identified more than 260 genes that statistically increase or decrease the relative risk of schizophrenia.[ref] Additionally, copy number variants and rare mutations can have large effects on schizophrenia risk.[ref]
It’s important to understand that none of the more common genetic variants (in the Genotype report section below) causes schizophrenia on its own. Instead, schizophrenia is a complex condition with many variables, environmental factors, and genetic variants coming together to change the way the brain works. People can have all of the SNPs that increase the risk of schizophrenia without ever having schizophrenia.
Studying these genetic underpinnings helps researchers understand brain changes at cellular and molecular levels. By knowing which genes are involved, researchers can develop solutions for schizophrenia – perhaps even solutions tailored to an individual’s genetic makeup.
Rare mutations affecting neuronal synaptic structure, ion channels, neuronal excitability, and transcriptional regulation have been identified as risk factors. Several mutations in glutamate receptors and signaling pathways suggest decreased NMDA or glutamate receptor function may be involved in some cases of schizophrenia, at least for some people. [ref]
Here are some of the genes that are both well-studied and validated in multiple studies.
NRG1 (Neuregulin 1):
In the brain, NRG1 is involved in the development of the nervous system, synaptic plasticity, and neuronal survival. Genetic variants in NRG1 are linked to low levels of white matter in certain areas of the brain. Balance is key. Animal models also show that overexpression of NRG1 can cause schizophrenia-like behavior. [ref][ref]
DISC1 gene (disrupted in schizophrenia 1):
This was the first gene identified decades ago as being associated with schizophrenia. DISC1 influences adult neurogenesis in the dentate gyrus region of the brain. Recent research shows that DISC1 may also be involved in neural development in other ways, such as synaptic transmission and the development of astrocytes.[ref] Interestingly, DISC1 interacts directly with GSK3B, which is also involved in bipolar disorder and circadian rhythm.[ref] DISC1 also modulates the transcription factor ATF4, which is “involved in the regulation of cellular stress responses, emotional behaviour and memory consolidation.” ATF4 is usually found at low levels, but when a stressor occurs, ATF4 ramps up and then increases or decreases the transcription of other genes involved in cellular stress. Specifically, ATF4 increases autophagy in the neurons when cellular stress happens, and DISC1 keeps that process under control. Thus, the variants that decrease DISC1 (and are linked to schizophrenia) allow more ATF4 and more autophagy in the neurons.[ref]
Complement factor 4 (C4):
The complement system is a part of the innate immune system that enhances the ability of antibodies and immune system cells to clear out pathogens and damaged cells. A number of genome-wide studies over the past decade showed that there was likely the involvement of either the HLA genes or the C4 genes in the risk of schizophrenia. More recently, researchers have narrowed it down to C4 as a causative factor in schizophrenia.[ref]
Brain studies on people with schizophrenia show a loss of gray matter without cell death due to reduced synaptic structures on neurons in specific brain regions. The brain’s immune response includes microglia, which can clear pathogens as well as prune synapses from neurons. Researchers think that overactivation of the complement system (C4A, specifically) during the final years of brain maturation (late adolescence, early adulthood) causes the pruning of synapses and changes to the networks in the brain.[ref]
RELN (Reelin):
RELN is an extracellular matrix protein found in many regions of the brain. It is important in the development of the cerebral cortex and helps neurons migrate during brain development. In the developed brain, RELN is produced by GABAergic neurons and is involved in synaptic plasticity and cognitive function. Genetic studies link mutations and common variants in RELN to schizophrenia and autism.[ref]
Related article: Reelin genes and brain health
Dopamine-related genes:
Dopamine is produced as a neurotransmitter, released into the synapse, and then taken up by dopamine receptors on nearby neurons. Genetic variants related to the amount of dopamine and the dopamine receptor functions are associated with an increased relative risk of schizophrenia. The COMT and MAO enzymes also can act on dopamine to break it down so that it is no longer an active neurotransmitter.[ref]
Related articles: Dopamine | COMT | MAO
Glutamate and GABA-related genes:
Glutamate is the excitatory neurotransmitter, and it is also the precursor molecule that is converted into GABA. Glutamate acts by binding to receptors, including the NMDA receptors. Variants in the NMDA receptors also increase susceptibility to schizophrenia.
Related articles: Glutamate synthesis | Glutamate receptors | GABA
Environmental factors involved in the initiation of schizophrenia:
Environmental factors that may trigger schizophrenia in genetically susceptible individuals include:
- Severe childhood stress or trauma
- Immune system dysregulation
- Cannabis use, especially in adolescence
- Prenatal toxin exposure or viral infections
Let’s look at some of these in more detail:
Inflammation and immune response: does it play a causal role?
Evidence suggests that inflammation may play a causal role in the predisposition and onset of schizophrenia. Many studies link elevated inflammatory cytokines to schizophrenia, but the question has always been whether inflammation causes psychosis or if schizophrenia is causing inflammation.
Let’s look at some of the research:
Epidemiological studies link prenatal, maternal, and early-life infections to an increased risk of schizophrenia.
Toxoplasma gondii:
Prenatal maternal infection with Toxoplasma gondii during pregnancy is linked in many studies to an increased risk of schizophrenia. A 2023 study using UK Biobank data showed that antibodies to T. gondii significantly increased the risk of schizophrenia developing.[ref][ref][ref][ref]
T. gondi is the parasite that causes toxoplasmosis. This is why pregnant women are told not to clean the cat’s litter box.
Viral infections:
Response to viral infections is another immune system link in schizophrenia. Studies on people with schizophrenia show higher antibody titers for cytomegalovirus, Epstein-Barr virus, and herpes simplex viruses.[ref]
Maternal viral infection:
A number of studies have shown that maternal viral infections are a risk factor for both schizophrenia and major affective disorder. This was first discovered when a correlation between schizophrenia in offspring born to mothers who were sick in the 1957 influenza epidemic. Animal studies clearly show that maternal infection during specific stages of brain development affects neurodevelopment.[ref]
Altered immune response:
Genetic studies have identified a number of variants in immune response genes that are risk factors for schizophrenia.[ref]
For example, complement factor C4 is activated as part of the innate immune response to pathogens, including T. gondii. C4 levels are elevated in people with schizophrenia, and genetic variants in C4 are linked to increased susceptibility.[ref]
Nutrition and childhood maltreatment:
The developing brain both prenatally and during early childhood is vulnerable to stressors and infections. Prenatal maternal stress increases the relative risk of psychiatric disorders in their children. Maternal smoking and high homocysteine in the mother are both slight risk factors.[ref]
Nutrition during infancy is critical for brain development. A clinical trial found that vitamin D supplementation during the first year of life (in Finland) greatly decreased the risk of developing schizophrenia as a young adult.[ref] Animal studies show that choline is likely also important in preventing schizophrenia.[ref][ref]
Drug use at a young age:
Cannabis use, especially frequent use in adolescence, has been identified as a risk factor for schizophrenia.[ref] Population-wide, cannabis use doubles the risk of schizophrenia.[ref] Other drugs, such as LSD and MDMA, are also associated with schizophrenia.[ref]
However, there has always been a chicken-or-the-egg question of whether people with schizophrenia are more likely to self-medicate with marijuana – or – whether THC specifically increases the risk of developing schizophrenia.[ref] A 2019 study looked at cannabis use alongside a polygenic risk score for schizophrenia(e.g. having more genetic susceptibility). The results showed that the higher genetic risk interacted with cannabis to make individuals more sensitive to the effects of environmental exposures.[ref]
A 2018 Mendelian randomization study showed that there was “evidence for a causal positive influence of schizophrenia risk on cannabis use.”[ref]
Epigenetics and schizophrenia:
DNA gives us the blueprint for proteins and enzymes, and genetic variants change that blueprint for the way that a protein or enzyme works (or doesn’t work).
DNA –> mRNA –> protein
But this is only part of what goes on in a cell…
Epigenetics refers to the multiple ways that genes can be turned on or off for transcription. Once a gene is transcribed into mRNA, there may be modifications that prevent it from being translated into its protein. Plus, once it is translated into a protein, there are post-translational modifications that can change the protein in ways that make it inoperable.
Researchers have found that transcriptional regulation – histone modification or DNA methylation – may play a role in schizophrenia. DNA methylation involves the addition of a methyl group to certain regions of DNA in the nucleus. The addition of the methyl group blocks the gene from being translated into protein. To add further complexity, genetic variants in non-coding regions can cause changes in the way genes are translated into their proteins.[ref]
Prenatal transcriptional changes and epigenetic changes in the immune response due to maternal infections or maternal immune system challenges are also associated with the risk of schizophrenia in their offspring.[ref]
Let’s take a look at how this plays out in people with schizophrenia:
I explained above that RELN genetic variants increase the risk of schizophrenia and that one epigenetic mechanism for silencing genes is methylation. Researchers have found that RELN DNA methylation levels are elevated in people with chronic schizophrenia and those at high risk for schizophrenia. This means that in addition to people with RELN gene variants, people with increased RELN methylation are also at higher risk for schizophrenia.[ref][ref]
Schizoaffective Disorder:
As with many health conditions, the list of symptoms and diagnostic criteria for schizophrenia doesn’t fit everyone perfectly. Everyone is unique – from their genes to their environmental exposures – and symptoms may fall on a spectrum or in multiple categories. This makes diagnosis more difficult when someone has symptoms of a mood disorder (MDD or bipolar) along with episodes of psychosis (delusions and hallucinations).
Schizoaffective disorder is a diagnosis that includes both mood symptoms and psychotic symptoms. The DSM-5 diagnostic criteria shifted to show schizoaffective disorder as a lifelong illness rather than an episodic diagnosis. It states: ” In the DSM-5, the diagnosis of Schizoaffective Disorder can be made only if full Mood Disorder episodes have been present for the majority of the total active and residual course of illness, from the onset of psychotic symptoms up until the current diagnosis.”[ref]
Some researchers consider schizoaffective disorder to be a variant of schizophrenia with prominent mood symptoms, while others consider it to be a severe form of major depression or bipolar disorder with episodes of psychotic symptoms.[ref]
What do genetic studies show for schizoaffective disorder? Well, genetic studies for either schizophrenia or bipolar disorder often include people with a diagnosis of schizoaffective disorder, which can make it hard to differentiate. Heritability studies using twins, siblings, and adopted twins show heritability of about 80% for schizophrenia, bipolar disorder, and schizoaffective disorder. Genome-wide association studies show some overlap between the disorders, such as CACNA1C and ANK3.[ref]
Genotype report: Schizophrenia-related variants
Lifehacks:
First, another disclaimer: Schizophrenia and schizoaffective disorders are serious mental health conditions. Please talk with your doctor before making any changes involving supplements, diet, or lifestyle.
My two takeaways from the research:
First, the two-hit hypothesis of genetic vulnerability along with an environmental ‘hit’ makes me aware of how important it is to ensure that kids have a healthy childhood and adolescence. Malnutrition, stress, maltreatment, and sexual abuse are all linked to increased susceptibility to schizophrenia. Drug use in the teen years when the brain is still developing is also an environmental factor. Be good to your kids and provide a healthy environment for their brains during development.
Second, while schizophrenia is a serious, life-long condition, there are quite a few clinical trials showing ways to improve symptoms alongside prescription medications. Several of these trials point towards reducing neuroinflammation or improving neurotransmitter receptor function. What is listed here is just the tip of the iceberg as far as the clinical trials that have been done. I encourage you to work with your doctor or healthcare team to improve any continuing symptoms.
Immune response:
Treating viral infections:
In schizophrenia patients who were cytomegalovirus antibody positive, treatment with valacyclovir (anti-viral) improved psychiatric symptoms.[ref] Another study showed that valacyclovir reduced neuroinflammation as seen on a CT scan in schizophrenia.[ref] For patients who were also seropositive for herpes simplex virus 1, valacyclovir improved verbal memory, working memory, and other cognitive scores.[ref]
Anti-parasitic medications:
Animal studies show that antiparasitic medications that treat toxoplasmosis change behavior and neurocognitive function.[ref]
Gut microbiome:
The gut microbiome continually interacts with your immune system. While much of the research here is still fairly new, studies show that there are significant changes in the gut microbiome in people with schizophrenia.[ref][ref][ref][ref] Transplanting the gut microbiome from patients with schizophrenia into mice causes schizophrenia-like behavior in the mice.[ref][ref] Talk with your doctor about your options for changing your gut microbiome through diet or FMT.
Exercise programs:
Exercise helps to modulate immune response as well as having other brain benefits. Clinical trials involving exercise programs show that it helps with schizophrenia symptoms, although the dropout rate was high. One study showed that either aerobic exercise or strength and flexibility training helped.[ref][ref]
Diet, Vitamins, and Nutrients:
A healthy diet has been shown in multiple studies to help with managing and mitigating symptoms in schizophrenia and schizoaffective disorder patients.[ref][ref] However, just taking a mega-vitamin doesn’t do much for symptoms.[ref]
Let’s look at some of the more specific dietary and nutrient interventions:
Related Articles and Topics:
Glutamate: Synthesis, transport, and supplement interactions
Dopamine Synthesis SNPs: Genes, lifestyle, diet, and dopamine optimization
COMT: How to Optimize Your Supplements for Your COMT Genotype
References:
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.