Key takeaways:
~ The 2016 book The SIRTfood Diet proposes weight loss occurs by activating sirtuins.
~ Sirtuins are cellular energy sensors that can turn on or off genes in response to how much energy is available.
~ Genetic variants in the SIRT genes can impact your risk for weight gain as well as healthy longevity.
~ The Lifehacks section will explain the scientific research on different sirtuin activators and how you could apply these solutions to ‘hack’ your sirtuins for healthy aging and increased metabolism.
Sirtuins: background and overview
Sirtuins are a family of seven proteins that are important in removing acetyl groups from molecules.[ref]
What are acetyl groups, and why should I care? Acetyl groups within the nucleus of the cell attach to the DNA at certain points, marking and opening up the DNA for transcription. Think of them as a chemical post-it note, pointing to what needs to be transcribed into a protein or enzyme for the cell.
Sirtuins remove the acetyl group at the right time, allowing the DNA to compact again and protect it from damage. Essentially, sirtuins turn off a protein. This is an essential function to regulate proteins that are important for aging, metabolism, circadian rhythm, and cell growth.
Sirtuins respond to the energy level changes in a cell. They regulate the transcription of other proteins based on how much energy is available.
In addition to removing acetyl groups in the cell nucleus, some sirtuins are active in the mitochondria and the cytoplasm of the cell. These mitochondrial sirtuins are important in energy production, metabolism, and cellular health.
Let’s take a deeper dive into several sirtuin genes:
SIRT1: Energy sensor, metabolism, and adipose tissue
The SIRT1 protein is located in all cells. Its job in the nucleus of the cell is to remove the acetyl groups that mark which genes should be translated into their proteins. Thus, SIRT1 influences when other proteins are made by the cell. It’s a regulator reacting to the energy available in the cell.[ref]
Essentially, when cellular energy is low, SIRT1 is one way some proteins are turned off to conserve cellular energy.
SIRT1 and metabolism:
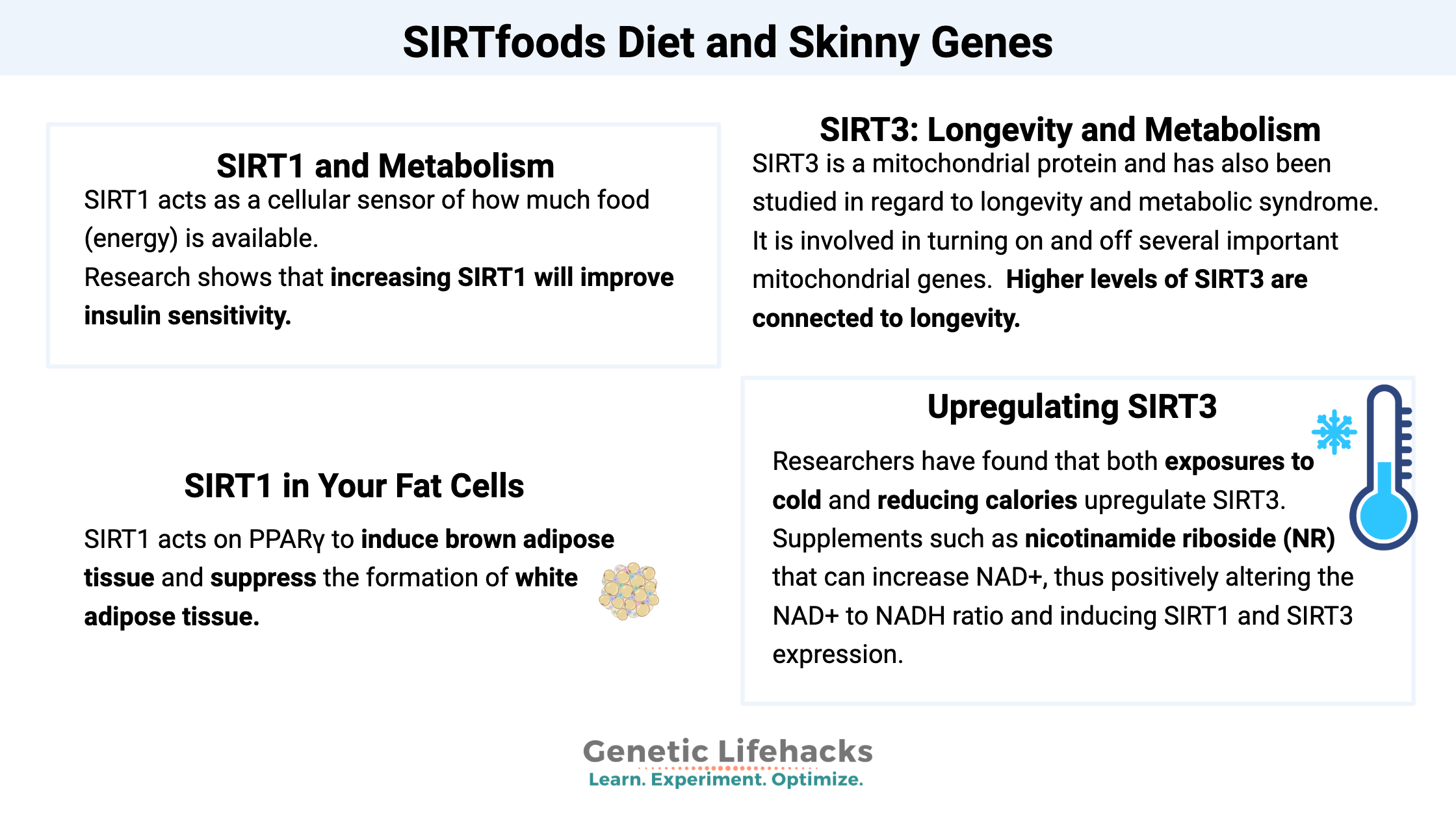
SIRT1 acts as a cellular sensor of how much food (energy) is available. At a cellular level, the level of NAD+ is one-way SIRT1 is activated. NAD+ is created and used in the mitochondria during energy production. When energy is low, such as during calorie restriction or hard exercise, the ratio of NAD+ to other mitochondrial molecules rises. This increase in NAD+ triggers an increase in SIRT1 expression.
By affecting the expression of different proteins involved in metabolism, circadian rhythm, and autophagy, SIRT1 affects both overall metabolic rate (and weight) as well as longevity.
Insulin resistance causes SIRT1 levels to decrease. Research shows that increasing SIRT1 will improve insulin sensitivity, especially in situations of insulin resistance. SIRT1 does this by repressing the translation of a gene called PTP1B, a protein tyrosine phosphatase that is a regulator of insulin signaling.[ref]
Calorie restriction (40% fewer calories than normal) increases SIRT1. It is thought to be a mechanism by which calorie restriction increases lifespan in some animals.[ref]
SIRT1 in your fat cells:
Adipose tissue (fat cells) can be either white or brown.
- White adipose tissue, what we think of as fat, is found in areas throughout the body (you know – the belly, love handles, thighs, etc.).
- Brown adipose tissue, or brown fat, is a good kind of fat that burns through a lot of energy. It keeps babies warm, producing heat without shivering.
SIRT1 acts on PPARγ to induce brown adipose tissue and suppress the formation of white adipose tissue.[ref]
SIRT1 and cancer:
As we age, one thing to keep in mind is the balance between the needed cellular regeneration and prevention of the out-of-control growth of cancerous cells.
While it may seem like we always want to increase SIRT1, there could be trade-offs when it comes to preventing a cancerous cell from being destroyed.
In regards to autophagy, one important gene that SIRT1 acts upon is called p53. This is a tumor suppressor gene that needs to be available in cells in the right amount to cause cell death in a cancerous cell. The p53 protein stops the process of cell division, which allows for either a repair of the damaged DNA or apoptosis (cell death).
There are several animal and cell studies showing that inhibiting SIRT1 may help to combat cancer.[ref]
Don’t get me wrong here. I am not suggesting the SIRTfood diet will promote cancer. Instead, I want to clarify that if you have cancer, be cautious and thoroughly investigate supplements that increase SIRT1 activity.[ref] Talk with your doctor, of course.
SIRT3: Longevity and Metabolism
SIRT3 is a mitochondrial protein and has also been studied in regard to longevity and metabolic syndrome. It is involved in turning on and off several important mitochondrial genes. Higher levels of SIRT3 are connected to longevity.
SIRT3 and metabolism:
SIRT3 is also involved in metabolism, and a study with mice lacking SIRT3 had greater obesity and insulin resistance on a high-fat diet. The reduction of the SIRT3 function was found to lead to mitochondrial dysfunction and metabolic syndrome.[ref][ref]
SIRT3 regulates the activity of proteins that are important for mitochondrial function, such as those needed for fatty acid oxidation and for reducing oxidative stress.[ref]
Researchers have found that both exposures to cold and reducing calories upregulate SIRT3.[ref]
SIRT3 function is also important in preventing cancer. Mice with the gene deleted are more likely to have tumors, and human breast cancer tissue shows deletion of SIRT3 in 40% of carcinomas.[ref]
A December 2014 study in Cell Metabolism found that noise-induced hearing loss in mice could be prevented with nicotinamide riboside, which is a precursor to NAD+ (nicotinamide adenine dinucleotide) and a derivative of vitamin B3. [ref] The researchers found nicotinamide riboside activated SIRT3 in the mitochondria. This increase in SIRT3 prevented noise-induced hearing loss. Moreover, the addition of nicotinamide riboside (NR) was effective either before or after hearing loss.[ref]
Other studies show calorie restriction slows age-related hearing loss through increasing SIRT3 “by promoting the glutathione-mediated mitochondrial antioxidant defense system.”[ref][ref]
SIRT1 acts on SIRT3: post-translational modification
Interestingly, SIRT1 acts to deacetylate SIRT3 in a post-translational manner. I mentioned above that acetylation marks a gene to be translated into a protein, but that is only part of the picture. Acetyl groups also act upon proteins after they have been created, and acetylation or deacetylation of a protein can change the way it functions in a cell.[ref]
In the case of SIRT3, the SIRT1 protein can regulate SIRT3 activity and mitochondrial function.[ref]
Why is this important? We often think of changing one gene to alter one function – like pulling a lever to turn something on or off. But it can often be more complicated than just that single lever; the interactions between the sirtuins may also be important in overall metabolism.
SIRT6: Mitochondrial energy, gluconeogenesis, and cancer prevention
SIRT6 also functions in mitochondrial energy production.
One role of SIRT6 is to help regulate the production of glucose in the liver during fasting. Called gluconeogenesis, the liver produces glucose to regulate blood glucose levels. SIRT6 interacts with several other proteins in turning off and on genes that regulate liver glucose production. Animal models of obesity and diabetes have reduced SIRT6 levels.[ref]
Additionally, SIRT6 senses a type of DNA damage called double-strand breaks, which are dangerous to a cell. SIRT6 activates the DNA damage response to repair damage to your chromosomes.[ref][ref]
NAD+ and Sirtuins:
I mentioned above that the way SIRT1 detects a change in cellular energy levels is through the change in the ratio of NAD+ to NADH.
Related article: In-depth article on NAD+ here.
The ratio of NAD+ to NADH changes when there are stresses to the cell. This can include a lack of food (calorie restriction for a few days), hard exercise, and also oxidative stress. Through sensing the changes in NAD+, the sirtuins act as stress adaptors.[ref]
Alternatively, there are supplements such as nicotinamide riboside (NR) that can increase NAD+, thus positively altering the NAD+ to NADH ratio and inducing SIRT1 and SIRT3 expression.[ref][ref]
SIRT Genotype Report
Access this content:
An active subscription is required to access this content.
Lifehacks:
The SIRTfood diet starts with a low-calorie (1000 – 1500 calories) induction week coupled with drinking lots of green smoothies. Then the diet plan moves through several phases with specific food recommendations.
Let’s take a look at how this stacks up to what research studies show.
The rest of this article is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
Metformin
A decades-old diabetes drug now holds promise for increasing healthspan. Research shows that metformin may reduce the risk of some of the diseases of aging, thus increasing the number of years someone is healthy.
Vitamin D, Genes, and Your Immune System
Vitamin D is more than just a ‘vitamin’. It is actually a hormone that is essential to so many processes in your body – including your immune system. This article explains how vitamin D interacts with the immune system and which genetic variants are linked with low vitamin D levels. We will wrap up with ways to increase your vitamin D level.
Lithium Orotate: Mood, Alzheimer’s Prevention, and Telomeres
Lithium is a naturally occurring mineral that we consume in small amounts each day. Research now shows that low doses of lithium may impact mood, Alzheimer’s disease, and telomere length.