Key takeaways:
~ Tendinitis is due to disorganized collagen bundles in the tendon.
~ Chronically elevated inflammatory cytokines cause the remodeling of the tendon to be disrupted.
~ Understanding where your genetic susceptibility lies can help you target the right solutions to heal – and prevent – tendinitis.
What is tendonitis (tendinitis), and why does it happen?
Do you have problems with your tennis elbow, rotator cuff, knees, or Achilles tendon? If so, you’ve probably followed the usual advice of rest, ice, and keeping it immobile. Then you wait and hope that it heals. One of the reasons there are few, if any, effective treatments for tendinopathy is a lack of knowledge regarding its pathogenesis. In other words, we didn’t know much about how tendinopathy works until recently.
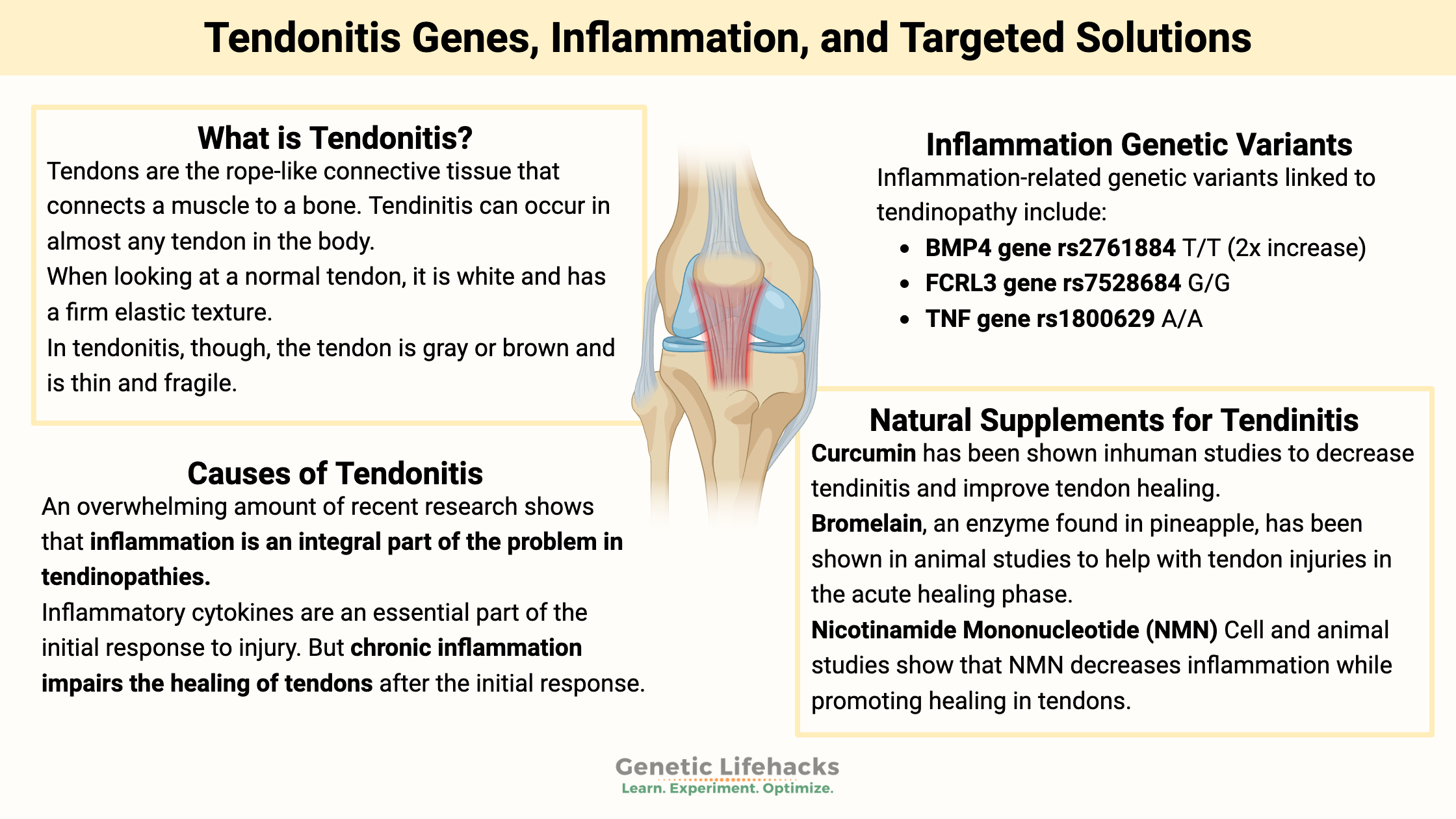
Tendons are the rope-like connective tissue that connects a muscle to a bone. They are the connections that move the joint, transmitting forces from the muscle to the bone.
Tendon injuries are common and can include:
- tendinitis (or tendinopathy) is an inflamed tendon
- torn tendons
Tendinitis can occur in almost any tendon in the body, and many different terms refer to these issues. Examples include:
- tennis elbow or golfer’s elbow
- plantar fasciitis (bottom of the foot)
- Achilles tendonitis (ankle)
- trigger finger (tendonitis in the index finger)
- rotator cuff injury (shoulder)
- patellar (knee) tendonitis
There seems to be a lack of good treatment options for such common injuries. The Cleveland Clinic recommends: rest, ice, taking NSAIDs, and seeing your doctor if it still hurts in three weeks.[ref]
A research study on tendon injuries explains:[ref]
“One of the reasons there are very few, if any, effective treatments for tendinopathy is lack of knowledge regarding its pathogenesis.”
So let’s dig into the ‘pathogenesis’, or the underlying changes in tendon injuries, and see what is causing the problems.
Changes seen in tendinitis:
When looking at a normal tendon, it is white and has a firm elastic texture. Tendons are made up of parallel and organized collagen bundles.
In tendonitis, though, the tendon is gray or brown and is thin and fragile. The collagen bundles that make up the tendon are disorganized, meaning that under a microscope, they show up as varying in diameter and not oriented in the same direction. Healthy tendons don’t have a lot of blood flow, but in tendonitis, the tendon has ingrowths of small blood vessels and small nerves.[ref]
Here’s an image showing how the collagen fibers should be organized in a tendon:
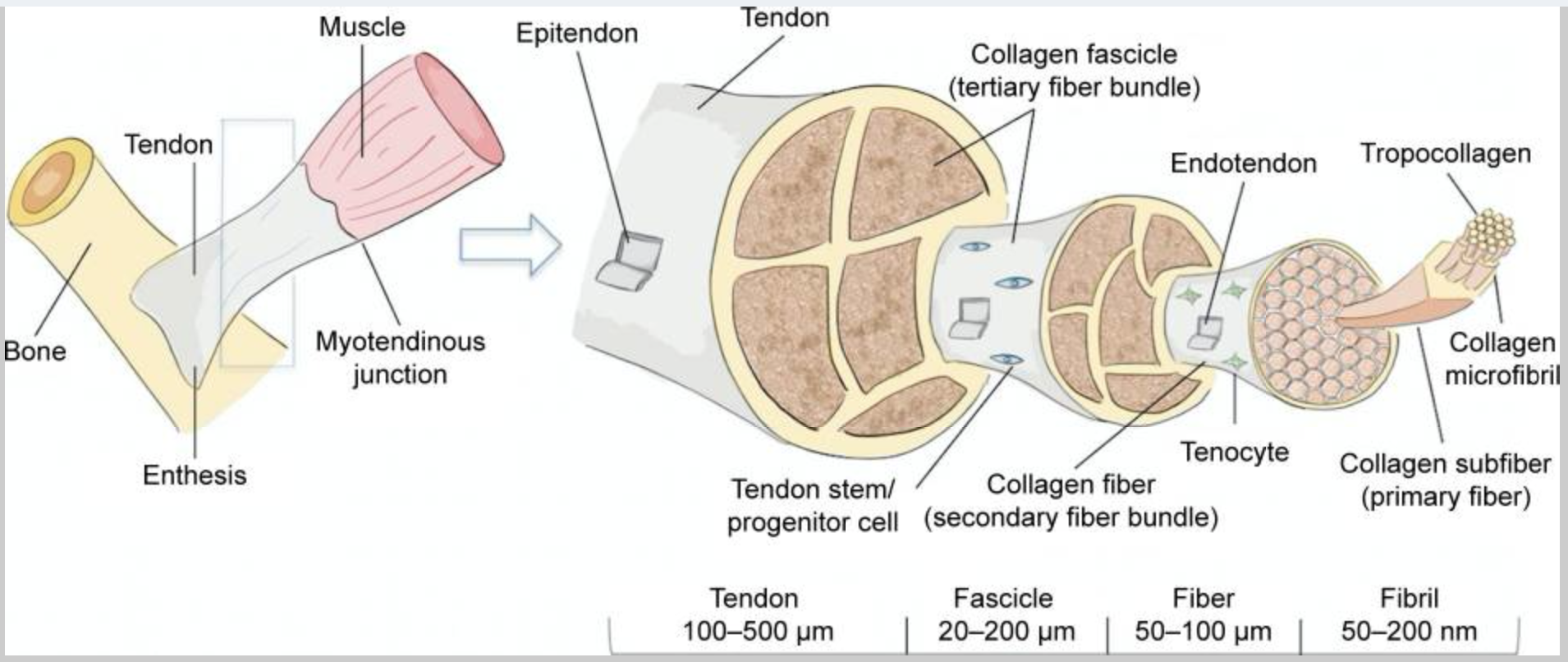
Let’s take a closer look at the components of healthy tendons:
Extracellular matrix, collagen, and tendon structure:
The extracellular matrix (ECM) surrounds and supports cells in your body. The ECM consists of the following:
- structural proteins (collagen, elastin)
- specialized proteins (fibrillin, fibronectin)
- proteoglycans
The ECM is vital in the composition of the tendon, giving the structure around the tendon cells. Tendon cells are a specialized type of fibroblast, which are cells that produce the extracellular matrix.
Collagen in the extracellular matrix is what makes up most of the tendon. There are a bunch of types of collagen, but the primary ones in tendons are type I, type III, and type IV. In addition to collagen, the ECM in tendons also contains a little cartilage, elastin, and proteoglycans.
Proteoglycans are proteins bound to strings of carbohydrate molecules. In the ECM, proteoglycans help increase the tendon’s water content, which helps to resist compression.[ref] In my mind, proteoglycans are like jello, keeping the tendon spongy yet firm.
The type of polysaccharide (carbohydrate) attached to proteoglycans is glycosaminoglycans. It may sound familiar to anyone looking at glucosamine supplements marketed for joint health.
Remodeling of tendons: dynamic and continuing.
The extracellular matrix in tendons isn’t a static, fixed thing. A tendon is thought to be rope-like, but there is constant turnover and remodeling. Parts of the ECM are degraded and recycled, while at the same time, new ECM is synthesized.[ref]
Matrix metalloproteinases (MMP) are enzymes that completely degrade connective tissue and modify the extracellular matrix. Just as there are several types of collagen, several different MMP enzymes also break down collagen.
ADAMs (a disintegrin and metalloproteinase) are enzymes that can break down the non-collagen parts of tendons and signal for the assembly of collagen fibrils.
What happens when a tendon is injured?
Tendon repair occurs in stages, beginning with an inflammatory stage that lasts for a few days. The repair process then takes weeks to months to complete.
Growth factors are signaling molecules that are released in the area of an injury. IGF1 (insulin growth factor 1) and TGFβ (transforming growth factor beta) are activated almost immediately in an injury. They remain active throughout most phases of tendon healing.
Researchers think that IGF1 is signaling to stimulate new fibroblasts to the injury site and promote the production of the extracellular matrix during remodeling. PDGF (platelet-derived growth factor) is involved in the initial stages of the injury, and it initiates more collagen production as well as increases IGF1.
After the initial injury phase, VEGF (vascular endothelial growth factor) increases and helps to form new, small blood vessels that bring nutrients to the injured tendon.[ref]. Combinations of genetic variants in the genes that encode VEGF are linked to a greater risk of tendinopathy.[ref]
Repetitive use and cell death:
Tendon ruptures, such as tearing an Achilles tendon, rarely happen as an acute injury (even though it seems to happen that way). Instead, tendons that tear usually show prior remodeling, microtraumas, and disordered collagen.[ref] The tendons have had strain and stress with inflammation and remodeling before the tear occurs.
Apoptosis is the process of cell death that goes on regularly in the body as old cells need to be replaced by new cells. Research shows that repetitive strain causes increased apoptosis (cell death) of the fibroblasts responsible for creating the ECM.[ref] While apoptosis is a normal part of the cell lifecycle, excess apoptosis can be a problem.
Chronic inflammation in tendinitis:
Doctors used to think that inflammation wasn’t a part of the degeneration in tendinitis, but research over the last decade or so shows that this is simply not true. Instead, an overwhelming amount of recent research shows that inflammation is an integral part of the problem in tendinopathies.[ref]
- Animal studies show that injecting inflammatory cytokines into a tendon can cause reduced tensile strength and increased tears over the course of several months.[ref]
- Biopsies of Achilles tendons show that people with Achilles problems have chronic, non-resolving inflammation. The injured/torn Achilles showed higher levels of IL-8, NF-κB, interferon, and PTGS2. Researchers theorize that the key to preventing further Achilles injuries is ensuring inflammation resolution.[ref]. (More on this in the lifehacks section — as well as this article on the resolution of inflammation as an active process.)
Inflammatory cytokines are an essential part of the initial response to injury. But researchers are finding that overall chronic inflammation, such as due to lifestyle factors, impairs the healing of tendons after the initial response (first couple of days).[ref] In fact, tendon problems are more common in people with chronic inflammatory conditions, such as obesity or smoking.[ref][ref]
IL-1B, an inflammatory cytokine, and MMP-1, the matrix metalloproteinase that breaks down collagen, can both be produced by tenocytes, the tendon cells that create the extracellular matrix. This production initiates two different pathways:
- The increase in IL-1B causes a cascade of inflammatory events, such as activating NF-κB.
- Elevated MMP-1 breaks down collagen in the tendon faster than it is being produced.[ref]
As I mentioned above, matrix metalloproteinases (MMPs) are enzymes that can break down collagen. MMP-1, MMP-8, and MMP-13 can degrade type I and type III collagen. Genetic variants that increase MMP-1, -8, and -13 are linked to an increased risk of tendon problems.
Inflammation in the tendon due to repetitive strain increases PGE2 (prostaglandin E2). It causes a ‘double whammy’: Prostaglandin E2 increases MMP-1 (increasing collagen breakdown) and inhibits collagen synthesis.[ref]
Additionally, research shows that in torn or damaged tendons, there is an increase in inflammatory cell types such as macrophages, mast cells, T-lymphocytes, and natural killer cells.[ref]
Recently published research points to a protein called CTRP3 as potentially important in tendinopathies. Researchers are still determining all that CTRP3 (C1q/tumor necrosis factor (TNF)–related protein-3) does, but currently, the understanding is that it is the regulation of inflammation and cell growth, including in tendons. The new research found that CTRP3 is markedly upregulated in tendinopathies, pointing again toward the importance of inflammation in tendon problems.[ref]
Taken together, recent research suggests that chronically elevated inflammation plays a significant role in the pathogenesis of tendon problems.
The continuation of elevated inflammatory cytokines — coinciding with the lack of resolution of inflammation — leads to remodeling, breakdown, and eventually disorganized collagen fibers in the tendons.
Tendinitis Genotype Report:
Lifehacks:
Research on tendinopathy is extensive and ongoing. Below are just a few of the recent studies on ways to treat or prevent tendon issues.
Drugs that increase tendinopathy:
Medications can interact with the matrix metalloproteinase enzymes and increase collagen turnover in the tendon.
- Statins are known to increase the risk of tendon problems. One study found a 50% increase in relative risk of trigger finger and a ~40% increased risk of shoulder tendinopathy with statin use. Experiments showed an increased release of MMP-1 and MMP-13 with simvastatin, which weakened and disrupted the tendon matrix.[ref]
- Fluoroquinolone antibiotics also are linked to an increased risk of tendinopathy, with Achilles tendon ruptures being the most common problem. The onset of tendon problems can happen weeks to months after taking fluoroquinolones.[ref]
MMP inhibitors:
Just as some drugs can increase MMPs, other drugs may benefit by inhibiting collagen breakdown in tendons. (Natural MMP inhibitors are listed below in the supplements section).
- Doxycycline: The commonly used antibiotic, doxycycline, inhibits the matrix metalloproteinase. Animal studies show that doxycycline may help repair Achilles tendon injuries and improve collagen filament integrity.[ref]
Platelet-rich plasma injections:
A common treatment for tendon issues is injections of platelet-rich plasma. But does it work? A meta-analysis of 29 studies found that platelet-rich plasma injections were likely better than doing nothing (wait-and-see method).[ref]
Polyphenol-rich Diet:
A flavonoid-rich diet may help to reduce overall inflammation. Fresh fruits and vegetables, such as blueberries, red peppers, parsley, and citrus fruits, are suggested as good sources of flavonoids that may help with tendinitis.[ref]
Natural supplements for tendinitis:
The rest of this article is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Related Articles and Topics:
TNF-alpha: Inflammation and Your Genes
Do you feel like you are constantly dealing with inflammation? Joint pain, food sensitivity, etc.? Perhaps you are genetically geared towards a higher inflammatory response. Tumor necrosis factor (TNF) is an inflammatory cytokine that acts as a signaling molecule in our immune system.
NLRP3 Inflammasome, Genetics, and Chronic Inflammation
What makes people more susceptible to chronic inflammatory diseases? The root of the over-activation of inflammation for some people could be the NLRP3 inflammasome.
C-Reactive Protein Gene: Marker of Inflammation
Chronic inflammation is the driver of many common diseases such as heart attacks, diabetes, obesity, and autoimmune diseases. C-Reactive Protein is a marker of inflammation. Genetic variants can increase or decrease CRP levels.
Will statins give you muscle pain? What your genes can tell you
Statins are one of the most prescribed medications in the world. One side effect of statins is myopathy, or muscle pain and weakness. Your genetic variants are significant in whether you are likely to have side effects from statins.
References:
Abrahams, Yoonus, et al. “Polymorphisms within the COL5A1 3’-UTR That Alters MRNA Structure and the MIR608 Gene Are Associated with Achilles Tendinopathy.” Annals of Human Genetics, vol. 77, no. 3, May 2013, pp. 204–14. PubMed, https://doi.org/10.1111/ahg.12013.
Aiyegbusi, A. I., et al. “Bromelain in the Early Phase of Healing in Acute Crush Achilles Tendon Injury.” Phytotherapy Research: PTR, vol. 25, no. 1, Jan. 2011, pp. 49–52. PubMed, https://doi.org/10.1002/ptr.3199.
Aiyegbusi, Ayoola I., et al. “A Comparative Study of the Effects of Bromelain and Fresh Pineapple Juice on the Early Phase of Healing in Acute Crush Achilles Tendon Injury.” Journal of Medicinal Food, vol. 14, no. 4, Apr. 2011, pp. 348–52. PubMed, https://doi.org/10.1089/jmf.2010.0078.
Altinisik, Julide, et al. “The BstUI and DpnII Variants of the COL5A1 Gene Are Associated With Tennis Elbow.” The American Journal of Sports Medicine, vol. 43, no. 7, July 2015, pp. 1784–89. PubMed, https://doi.org/10.1177/0363546515578661.
Arnoczky, Steven P., et al. “Activation of Stress-Activated Protein Kinases (SAPK) in Tendon Cells Following Cyclic Strain: The Effects of Strain Frequency, Strain Magnitude, and Cytosolic Calcium.” Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, vol. 20, no. 5, Sept. 2002, pp. 947–52. PubMed, https://doi.org/10.1016/S0736-0266(02)00038-4.
Baroneza, José Eduardo, et al. “MMP-1 Promoter Genotype and Haplotype Association with Posterior Tibial Tendinopathy.” Gene, vol. 547, no. 2, Sept. 2014, pp. 334–37. PubMed, https://doi.org/10.1016/j.gene.2014.07.001.
Bishop, Julie Y., et al. “Smoking Predisposes to Rotator Cuff Pathology and Shoulder Dysfunction: A Systematic Review.” Arthroscopy: The Journal of Arthroscopic & Related Surgery: Official Publication of the Arthroscopy Association of North America and the International Arthroscopy Association, vol. 31, no. 8, Aug. 2015, pp. 1598–605. PubMed, https://doi.org/10.1016/j.arthro.2015.01.026.
Bolon, Brad. “Mini-Review: Toxic Tendinopathy.” Toxicologic Pathology, vol. 45, no. 7, Oct. 2017, pp. 834–37. PubMed, https://doi.org/10.1177/0192623317711614.
Briški, Nina, et al. “Association of the Matrix Metalloproteinase 3 (MMP3) Single Nucleotide Polymorphisms with Tendinopathies: Case-Control Study in High-Level Athletes.” International Orthopaedics, vol. 45, no. 5, May 2021, pp. 1163–68. PubMed, https://doi.org/10.1007/s00264-020-04684-w.
Buhrmann, Constanze, et al. “Curcumin Modulates Nuclear Factor ΚB (NF-ΚB)-Mediated Inflammation in Human Tenocytes in Vitro.” The Journal of Biological Chemistry, vol. 286, no. 32, Aug. 2011, pp. 28556–66. PubMed Central, https://doi.org/10.1074/jbc.M111.256180.
Busch, Franziska, et al. “Resveratrol Modulates Interleukin-1β-Induced Phosphatidylinositol 3-Kinase and Nuclear Factor ΚB Signaling Pathways in Human Tenocytes.” The Journal of Biological Chemistry, vol. 287, no. 45, Nov. 2012, pp. 38050–63. PubMed Central, https://doi.org/10.1074/jbc.M112.377028.
Chisari, Emanuele, et al. “Tendon Healing Is Adversely Affected by Low-Grade Inflammation.” Journal of Orthopaedic Surgery and Research, vol. 16, Dec. 2021, p. 700. PubMed Central, https://doi.org/10.1186/s13018-021-02811-w.
—. “Tendon Healing Is Adversely Affected by Low-Grade Inflammation.” Journal of Orthopaedic Surgery and Research, vol. 16, Dec. 2021, p. 700. PubMed Central, https://doi.org/10.1186/s13018-021-02811-w.
Cho, Yongsik, et al. “CTRP3 Exacerbates Tendinopathy by Dysregulating Tendon Stem Cell Differentiation and Altering Extracellular Matrix Composition.” Science Advances, vol. 7, no. 47, p. eabg6069. PubMed Central, https://doi.org/10.1126/sciadv.abg6069. Accessed 10 June 2022.
Corps, Anthony N., et al. “Inhibition of Interleukin-1β-Stimulated Collagenase and Stromelysin Expression in Human Tendon Fibroblasts by Epigallocatechin Gallate Ester.” Matrix Biology, vol. 23, no. 3, June 2004, pp. 163–69. ScienceDirect, https://doi.org/10.1016/j.matbio.2004.05.001.
Dakin, Stephanie Georgina, et al. “Chronic Inflammation Is a Feature of Achilles Tendinopathy and Rupture.” British Journal of Sports Medicine, vol. 52, no. 6, Mar. 2018, pp. 359–67. PubMed Central, https://doi.org/10.1136/bjsports-2017-098161.
de Araujo Munhoz, Francielle Boçon, et al. “Posterior Tibial Tendinopathy Associated with Matrix Metalloproteinase 13 Promoter Genotype and Haplotype.” The Journal of Gene Medicine, vol. 18, no. 11–12, Nov. 2016, pp. 325–30. PubMed, https://doi.org/10.1002/jgm.2934.
Del Buono, Angelo, et al. “Metalloproteases and Tendinopathy.” Muscles, Ligaments and Tendons Journal, vol. 3, no. 1, May 2013, pp. 51–57. PubMed Central, https://doi.org/10.11138/mltj/2013.3.1.051.
Eliasson, Pernilla, et al. “Statin Treatment Increases the Clinical Risk of Tendinopathy through Matrix Metalloproteinase Release – a Cohort Study Design Combined with an Experimental Study.” Scientific Reports, vol. 9, no. 1, Nov. 2019, p. 17958. PubMed, https://doi.org/10.1038/s41598-019-53238-7.
Henrotin, Yves, et al. “Curcuminoids and Boswellia Serrata Extracts Combination Decreases Tendinopathy Symptoms: Findings from an Open-Label Post-Observational Study.” Current Medical Research and Opinion, vol. 37, no. 3, Mar. 2021, pp. 423–30. PubMed, https://doi.org/10.1080/03007995.2020.1860923.
Hudek, Robert, et al. “Degenerative Rotator Cuff Tears Are Associated with a Low Omega-3 Index.” Prostaglandins, Leukotrienes, and Essential Fatty Acids, vol. 148, Sept. 2019, pp. 35–40. PubMed, https://doi.org/10.1016/j.plefa.2019.07.004.
Imran, Muhammad, et al. “Myricetin: A Comprehensive Review on Its Biological Potentials.” Food Science & Nutrition, vol. 9, no. 10, Aug. 2021, pp. 5854–68. PubMed Central, https://doi.org/10.1002/fsn3.2513.
Jiang, Dapeng, et al. “Curcumin Improves Tendon Healing in Rats: A Histological, Biochemical, and Functional Evaluation.” Connective Tissue Research, vol. 57, no. 1, 2016, pp. 20–27. PubMed, https://doi.org/10.3109/03008207.2015.1087517.
Lipman, Kelsey, et al. “Tendinopathy: Injury, Repair, and Current Exploration.” Drug Design, Development and Therapy, vol. 12, Mar. 2018, pp. 591–603. PubMed Central, https://doi.org/10.2147/DDDT.S154660.
Lopes, Lucas Rafael, et al. “Association of TNF-α -308G > A Polymorphism with Susceptibility to Tendinopathy in Athletes: A Case-Control Study.” BMC Sports Science, Medicine & Rehabilitation, vol. 13, no. 1, May 2021, p. 51. PubMed, https://doi.org/10.1186/s13102-021-00276-2.
Lui, Pauline Po Yee, and Patrick Shu Hang Yung. “Inflammatory Mechanisms Linking Obesity and Tendinopathy.” Journal of Orthopaedic Translation, vol. 31, Dec. 2021, pp. 80–90. PubMed Central, https://doi.org/10.1016/j.jot.2021.10.003.
Mueller, Anna-Lena, et al. “Modulation of Inflammation by Plant-Derived Nutraceuticals in Tendinitis.” Nutrients, vol. 14, no. 10, May 2022, p. 2030. PubMed Central, https://doi.org/10.3390/nu14102030.
Posthumus, Michael, et al. “Components of the Transforming Growth Factor-Beta Family and the Pathogenesis of Human Achilles Tendon Pathology–a Genetic Association Study.” Rheumatology (Oxford, England), vol. 49, no. 11, Nov. 2010, pp. 2090–97. PubMed, https://doi.org/10.1093/rheumatology/keq072.
Rahim, Masouda, et al. “Human Genetic Variation, Sport and Exercise Medicine, and Achilles Tendinopathy: Role for Angiogenesis-Associated Genes.” Omics: A Journal of Integrative Biology, vol. 20, no. 9, Sept. 2016, pp. 520–27. PubMed, https://doi.org/10.1089/omi.2016.0116.
Rees, Jonathan D., et al. “Tendons – Time to Revisit Inflammation.” British Journal of Sports Medicine, vol. 48, no. 21, Nov. 2014, pp. 1553–57. PubMed Central, https://doi.org/10.1136/bjsports-2012-091957.
Salles, José Inácio, Marcus Vinícius Amaral, et al. “BMP4 and FGF3 Haplotypes Increase the Risk of Tendinopathy in Volleyball Athletes.” Journal of Science and Medicine in Sport, vol. 18, no. 2, Mar. 2015, pp. 150–55. PubMed, https://doi.org/10.1016/j.jsams.2014.02.011.
Salles, José Inácio, Lucas Rafael Lopes, et al. “Fc Receptor-like 3 (−169T>C) Polymorphism Increases the Risk of Tendinopathy in Volleyball Athletes: A Case Control Study.” BMC Medical Genetics, vol. 19, July 2018, p. 119. PubMed Central, https://doi.org/10.1186/s12881-018-0633-6.
Screen, H. R. C., et al. “Tendon Functional Extracellular Matrix.” Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society, vol. 33, no. 6, June 2015, pp. 793–99. PubMed Central, https://doi.org/10.1002/jor.22818.
September, A. V., et al. “Variants within the COL5A1 Gene Are Associated with Achilles Tendinopathy in Two Populations.” British Journal of Sports Medicine, vol. 43, no. 5, May 2009, pp. 357–65. PubMed, https://doi.org/10.1136/bjsm.2008.048793.
Sobhani-Eraghi, A., et al. “The Effect of Doxycycline on Achilles Tendon Repair in a Rat Model.” Malaysian Orthopaedic Journal, vol. 14, no. 3, Nov. 2020, pp. 155–60. PubMed, https://doi.org/10.5704/MOJ.2011.024.
Stone, D., et al. “Cytokine-Induced Tendinitis: A Preliminary Study in Rabbits.” Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, vol. 17, no. 2, Mar. 1999, pp. 168–77. PubMed, https://doi.org/10.1002/jor.1100170204.
“Tendinopathy: Symptoms, Causes & Treatment.” Cleveland Clinic, https://my.clevelandclinic.org/health/diseases/22289-tendinopathy. Accessed 10 June 2022.
van der Vlist, Arco C., et al. “Which Treatment Is Most Effective for Patients with Achilles Tendinopathy? A Living Systematic Review with Network Meta-Analysis of 29 Randomised Controlled Trials.” British Journal of Sports Medicine, vol. 55, no. 5, Mar. 2021, pp. 249–56. PubMed Central, https://doi.org/10.1136/bjsports-2019-101872.
Wang, Chunguang, et al. “Association of Polymorphisms Rs1800012 in COL1A1 with Sports-Related Tendon and Ligament Injuries: A Meta-Analysis.” Oncotarget, vol. 8, no. 16, Apr. 2017, pp. 27627–34. PubMed, https://doi.org/10.18632/oncotarget.15271.
Wang, Yunjiao, et al. “Aspirin Inhibits Inflammation and Scar Formation in the Injury Tendon Healing through Regulating JNK/STAT-3 Signalling Pathway.” Cell Proliferation, vol. 52, no. 4, July 2019, p. e12650. PubMed, https://doi.org/10.1111/cpr.12650.
Xu, Yinghua, and George A. C. Murrell. “The Basic Science of Tendinopathy.” Clinical Orthopaedics and Related Research, vol. 466, no. 7, July 2008, pp. 1528–38. PubMed Central, https://doi.org/10.1007/s11999-008-0286-4.
Yamaura, Kohei, et al. “Antioxidant Effect of Nicotinamide Mononucleotide in Tendinopathy.” BMC Musculoskeletal Disorders, vol. 23, Mar. 2022, p. 249. PubMed Central, https://doi.org/10.1186/s12891-022-05205-z.
Zhang, Weizhong, et al. “Controlled Release of Curcumin from Curcumin-Loaded Nanomicelles to Prevent Peritendinous Adhesion during Achilles Tendon Healing in Rats.” International Journal of Nanomedicine, vol. 11, 2016, pp. 2873–81. PubMed, https://doi.org/10.2147/IJN.S103867.