Key takeaways:
~ Trimethylaminuria (TMAU) is caused by mutations in the FMO3 gene, leading to high levels of trimethylamine (TMA) with a distinctive odor.
~ TMA is produced by gut bacteria breaking down certain foods and is normally converted to TMAO by the FMO3 enzyme.
~ High TMAO levels are linked to heart disease, while high TMA levels cause the odor associated with TMAU.
~ Genetic variants in FMO3 are listed below in the genotype report.
~ Dietary changes, such as reducing choline and fish intake, and supplements like riboflavin can help manage TMAU.
FMO3 gene mutations cause trimethylaminuria (TMAU):
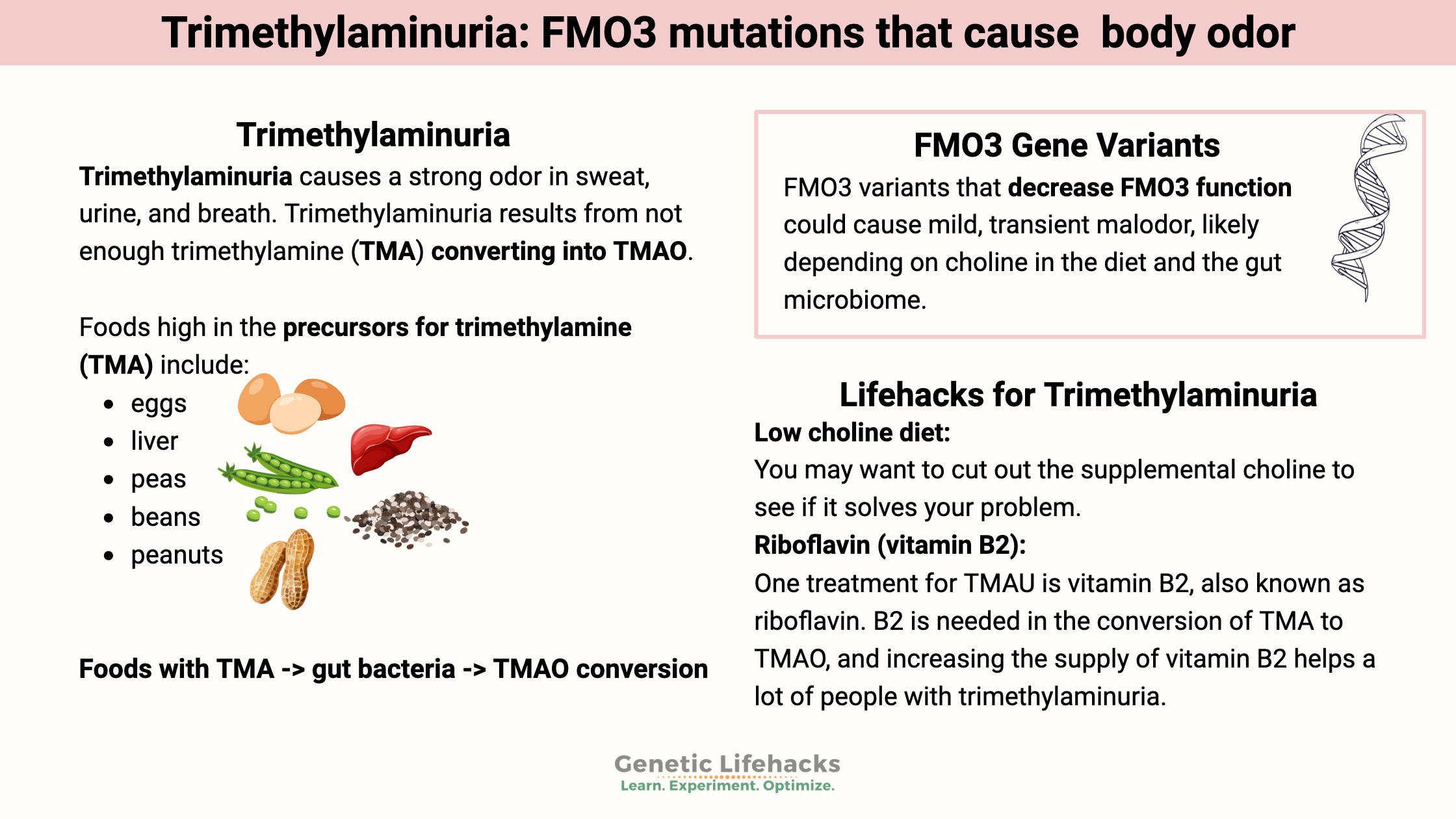
Trimethylaminuria is a metabolic disorder caused by high levels of trimethylamine, which has a distinctive odor. Often referred to as ‘fish odor disease’, trimethylaminuria causes a strong odor in sweat, urine, and breath.
Trimethylamine (TMA) forms in the intestines from various nutritional substrates when intestinal bacteria break down choline, carnitine, betaine, and ergothioneine. TMA then absorbs into the bloodstream from the intestines.[ref]
Foods with TMA -> gut bacteria -> TMAO conversion
Foods high in the precursors for trimethylamine (TMA) include:[ref]
- eggs
- liver
- kidney
- peas
- beans
- peanuts
- Brussels sprouts, broccoli, cabbage, cauliflower
- lecithin
- soy products
- some mushrooms (ergothioneine)
Gut bacteria act upon the foods containing the TMA precursors to produce trimethylamine. After TMA is absorbed in the intestines, the FMO3 enzyme converts it into TMAO (trimethylamine-N-oxide).[ref]
TMAO (trimethylamine-N-oxide) is the oxidized form of trimethylamine (TMA). Several problems linked to it include lipid homeostasis, diabetes, and heart health.
The drawback of high TMAO: A number of studies link higher plasma TMAO levels to an increased relative risk of thrombosis (heart attack, stroke). Platelets become a little bit more sticky – more easily activated to clump together – when levels of TMAO are high.[ref]
Too little FMO3 causes the opposite problem. Trimethylaminuria (a.k.a. fish odor disease) results from not enough trimethylamine (TMA) converting into TMAO.
Balance is critical here… you don’t want too much TMAO because of heart disease, but you also don’t want too much TMA due to the odor.
People with trimethylaminuria (TMAU) have genetic mutations in the FMO3 gene that lead to decreased enzyme function, resulting in trimethylamine (TMA) remaining in the bloodstream. The TMA has a bad (sometimes fishy) smell that can exude from the body.
Thus, we need just the right amount of TMA to TMAO conversion.
What are the signs and symptoms of trimethylaminuria?
The diagnosis of trimethylaminuria (TMAU) is based on a distinctive odor and high urinary TMA levels in comparison to TMAO.
The amount of TMA will vary over the course of the day, depending on what a person eats (e.g., choline or other TMA precursors). Thus, measuring the ratio is a better way to know how much TMA is being converted to TMAO. In general, a ratio of (TMAO/(TMAO + TMA)) is below 0.8 in people with mutations in the FMO3 gene.[ref]
The odor caused by TMAU smells like…
Most research studies refer to the body odor in trimethylaminuria (TMAU) as a ‘fishy odor’ or ‘rotten fish’, but patients with TMAU sometimes describe the odor in different ways. To some people, it could smell like garbage, urine, or rotting eggs. Plus, the presence of the odor can vary based on diet and gut microbiome composition.
A study of people with particularly malodorous BO found that about a third of them had TMAU.[ref]
Is trimethylaminuria always genetic?
Primary trimethylaminuria (TMAU) occurs due to a mutation that causes the FMO3 gene not to function well. Two copies of FMO3 mutations are generally needed to cause trimethylaminuria. Sometimes, though, a stronger odor is noticed in people with one copy of a mutation, depending on diet and the gut microbiome.
But…the cause of trimethylaminuria may not always be genetic.
While primary TMAU is a genetic disease caused by mutations in the FMO3 gene, a transient form is also possible.
The FMO3 enzyme is produced mainly in the liver. Adults can acquire TMAU if they have liver cirrhosis or viral hepatitis, which impairs liver function.
Transient TMAU can occur during menstruation or when consuming high amounts of choline (TMA precursor).
People with a combination of several FMO3 common genetic variants are more likely to have transient TMAU.[ref]
What else does the FMO3 enzyme do?
The FMO3 gene encodes an enzyme that is important in several reactions in the body (including the breakdown of TMAO).
Nicotine metabolism: Nicotine is mainly metabolized in the body using the CYP2A6 enzyme. But the FMO3 enzyme can also play a role in nicotine metabolism, especially in people who have genetic variants that cause low CYP2A6 activity. Additionally, people with genetic variants that slow the FMO3 enzyme function have an altered response to nicotine.[ref]
Related article: CYP2A6 genetic variants
Drug metabolism: Certain drugs, including tamoxifen and cimetidine, are metabolized using the FMO3 enzyme. Serious interactions can occur with FMO3 mutations and busulfan, which is used in stem cell transplants.[ref][ref]
FMO3 Genotype Report:
Access this content:
An active subscription is required to access this content.
Lifehacks for trimethylaminuria:
Below are some research-based diet and supplement recommendations that work for some people with TMAU. Talk with your doctor, of course, if you have been diagnosed with TMAU, to find out what is right for you.
Low choline diet:
If you have problems with transient malodorous BO and are taking choline supplements, you may want to cut out the supplemental choline to see if it solves your problem.
The first line of treatment for someone diagnosed with TMAU is often a low-choline diet. Choline is really important, though, for a lot of cellular functions. For a child with TMAU, the low choline diet should be supervised by a doctor and dietician because choline is vital in the growing brain. Choline is also essential for anyone planning on getting pregnant.
Limiting fish in the diet:
Salt-water fish contain TMAO. Consuming a lot of fish can lead to an alteration in the ratio of TMA to TMAO – sometimes resulting in too much TMA. For people with trimethylaminuria, a diet that avoids fish and seafood is recommended.[ref]
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
References
About Trimethylaminuria. https://www.genome.gov/Genetic-Disorders/Trimethylaminuria. Accessed 24 Mar. 2025.
Bouchemal, Nadia, et al. “Diagnosis and Phenotypic Assessment of Trimethylaminuria, and Its Treatment with Riboflavin: 1H NMR Spectroscopy and Genetic Testing.” Orphanet Journal of Rare Diseases, vol. 14, Sept. 2019, p. 222. PubMed Central, https://doi.org/10.1186/s13023-019-1174-6.
El-Serafi, Ibrahim, et al. “Flavin-Containing Monooxygenase 3 (FMO3) Role in Busulphan Metabolic Pathway.” PLoS ONE, vol. 12, no. 11, Nov. 2017, p. e0187294. PubMed Central, https://doi.org/10.1371/journal.pone.0187294.
Fennema, Diede, et al. “Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease.” Drug Metabolism and Disposition, vol. 44, no. 11, Nov. 2016, pp. 1839–50. PubMed Central, https://doi.org/10.1124/dmd.116.070615.
Gatarek, Paulina, and Joanna Kaluzna-Czaplinska. “Trimethylamine N-Oxide (TMAO) in Human Health.” EXCLI Journal, vol. 20, Feb. 2021, pp. 301–19. PubMed Central, https://doi.org/10.17179/excli2020-3239.
Perez-Paramo, Yadira X., et al. “Nicotine-N’-Oxidation by Flavin Monooxygenase Enzymes.” Cancer Epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, vol. 28, no. 2, Feb. 2019, pp. 311–20. PubMed Central, https://doi.org/10.1158/1055-9965.EPI-18-0669.
Schmidt, Aaron C., and Jean-Christophe Leroux. “Treatments of Trimethylaminuria: Where We Are and Where We Might Be Heading.” Drug Discovery Today, vol. 25, no. 9, Sept. 2020, pp. 1710–17. ScienceDirect, https://doi.org/10.1016/j.drudis.2020.06.026.
Shih, Diana M., et al. “Flavin Containing Monooxygenase 3 Exerts Broad Effects on Glucose and Lipid Metabolism and Atherosclerosis.” Journal of Lipid Research, vol. 56, no. 1, Jan. 2015, pp. 22–37. PubMed Central, https://doi.org/10.1194/jlr.M051680.
Shimizu, Makiko, et al. “Relationships between Flavin-Containing Mono-Oxygenase 3 (FMO3) Genotype and Trimethylaminuria Phenotype in a Japanese Population.” British Journal of Clinical Pharmacology, vol. 77, no. 5, May 2014, pp. 839–51. PubMed Central, https://doi.org/10.1111/bcp.12240.