Key takeaway:
~ Your circadian rhythm is a fundamental part of health and wellness.
~ Disruption to circadian rhythm gene expression is causal for chronic diseases
~ Genetic variants in core circadian genes can help you understand how and why circadian rhythm optimization may be important for you.
What is circadian rhythm?
Circadian Rhythms: The Unsung Heroes of Our Biological Symphony
Circadian rhythms are the intrinsic biological patterns that orchestrate our physiological processes. For example, the master clock in our brains regulates the sleep-wake cycle across a 24-hour period, governed by primary ‘clock’ genes.
Our biological molecular clock, called the core circadian clock, sets the rhythm of literally thousands of our genes over the course of about 24 hours.
Recent discoveries, however, extend beyond this central clock. Peripheral clocks exist in various organs like the liver, pancreas, and even in fat cells. These peripheral rhythms are crucial for the timely production of proteins in these organs.
The gut microbiome, too, participates in this rhythmic dance, exhibiting its own circadian fluctuations. Disruptions in our circadian rhythms can, in turn, upset this microbial balance, illustrating the interconnectedness of our body’s systems. [ref]
Diving into Chronobiology
The hypothalamus is the region of the brain that houses the suprachiasmatic nucleus (SCN), the conductor of our central circadian rhythm.
Environmental cues like sunrise, meal timing, temperature, and exposure to artificial lighting, termed ‘zeitgebers,’ play pivotal roles in synchronizing these rhythms.
Sunlight’s role is paramount in setting our core circadian rhythm. Specifically, short, blue wavelengths of sunlight interact with intrinsic photosensitive retinal ganglion cells in the eye, signaling the SCN and synchronizing our 24-hour cycle.
Until the advent of modern electric lights, particularly LED and fluorescent lights, the only time we were exposed to light in the blue wavelengths (around 450-480nm) was during daylight.
Two significant disruptors of circadian rhythms are nighttime blue light exposure (around 450-480nm) and insufficient morning sunlight. Coupled with individual genetic variants affecting clock gene functions, these factors underscore the complexity and importance of circadian rhythms.
The Genetic Machinery Behind Circadian Rhythms
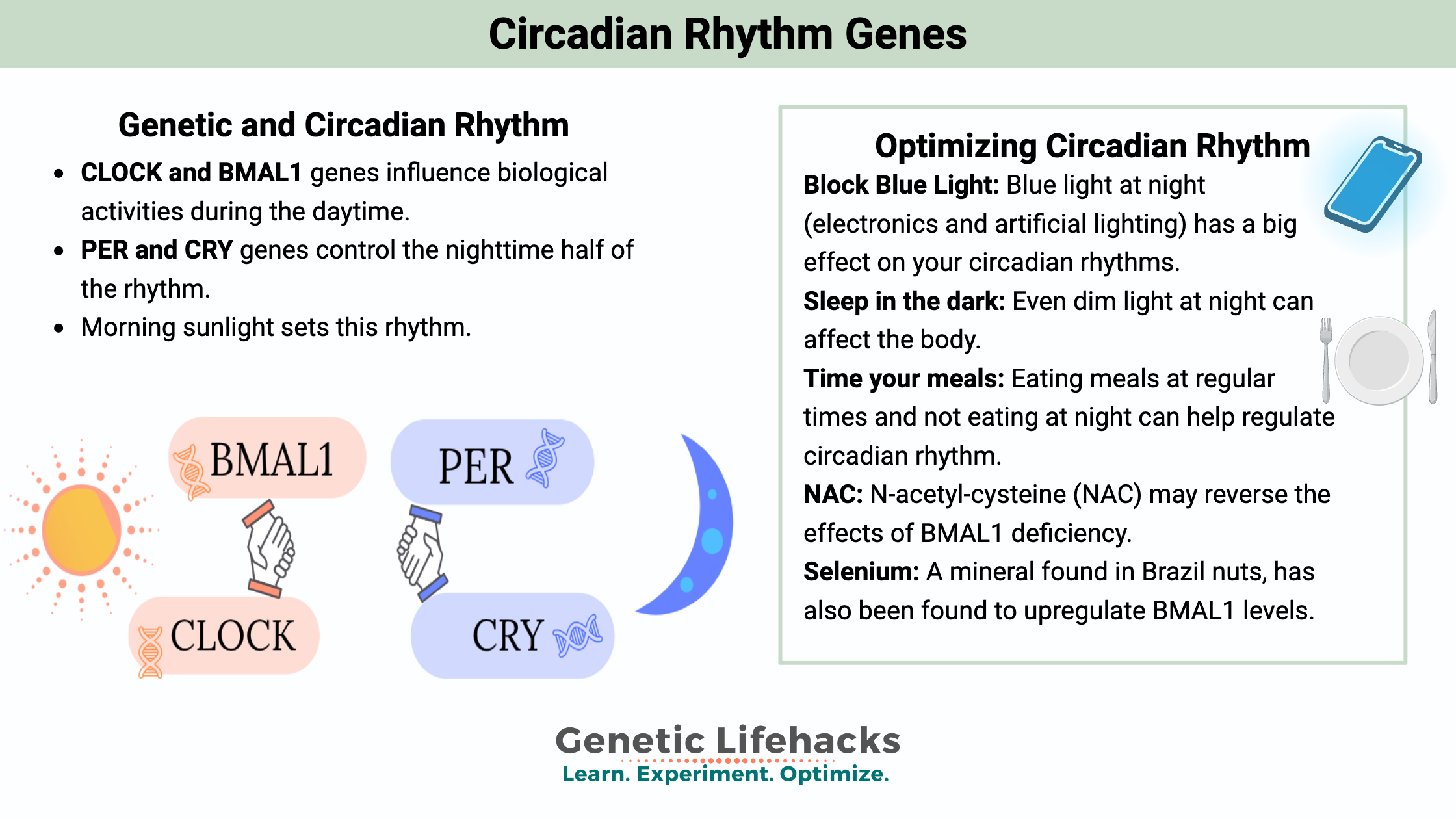
The core of circadian rhythm regulation lies in an intricate network of genes and their protein products. Central to this are the CLOCK and BMAL1 genes. These genes encode proteins that form a complex, initiating the transcription of other circadian genes, such as PER (Period) and CRY (Cryptochrome).
During the day, CLOCK and BMAL1 proteins bind together and promote the expression of PER and CRY genes. The proteins produced by these genes gradually accumulate in the cell’s cytoplasm and, as night approaches, they form a complex that moves back into the nucleus. This complex inhibits the activity of the CLOCK-BMAL1 complex, thereby reducing their own production in a negative feedback loop.
To simplify:
CLOCK and BMAL1, influence biological activities during the daytime.
PER and CRY control the nighttime half of the rhythm.
As PER and CRY proteins degrade over time, their inhibition on CLOCK-BMAL1 diminishes, allowing the cycle to start again. This feedback loop is approximately 24 hours, aligning with the Earth’s day-night cycle.
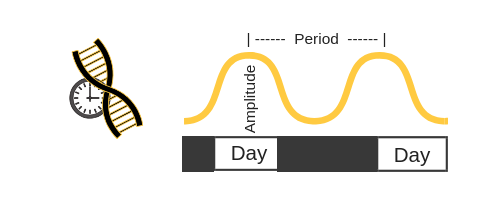
Period and Amplitude of Circadian rhythm:
When it comes to the circadian clock genes, researchers like to use terminology like ‘period’ and ‘amplitude’. Let’s take BMAL1 as an example: the levels will rise and then fall (amplitude) over the course of 24 hours (period).
It is easier to picture this if you visualize the amount of BMAL1 rising and falling like a sine wave:
Regulation by Post-Translational Modifications
Post-translational modifications of circadian proteins play a pivotal role in maintaining the precision of the circadian clock. Phosphorylation, for instance, regulates the stability and activity of circadian proteins. Kinases and phosphatases add and remove phosphate groups, respectively, influencing the timing of the feedback loop.
Setting the circadian clock.
For humans, the average circadian clock runs about 15 minutes longer than 24 hours. But we aren’t all average, and the circadian period varies between a few minutes less than 24 hours to about a half-hour longer.[ref].
To keep us on track, the body has a built-in clock setting mechanism — exposure to light.
Imagine if your clock ran at about 24.5 hours instead of 24. Within a week, you would be about seven hours out of sync.
They’ve done studies with people kept in caves or bunkers, and with no outside light, this does happen.
Below is a graph from a recent study where the participant stayed in a bunker for 9 days. The first two days and the last two days, he had lights available to turn on when he wanted. During days 3 through 7, a dim light was constantly turned on. As you can see, sleep timing (in yellow) and sleep duration changed a lot throughout the week. (CC image) [ref]
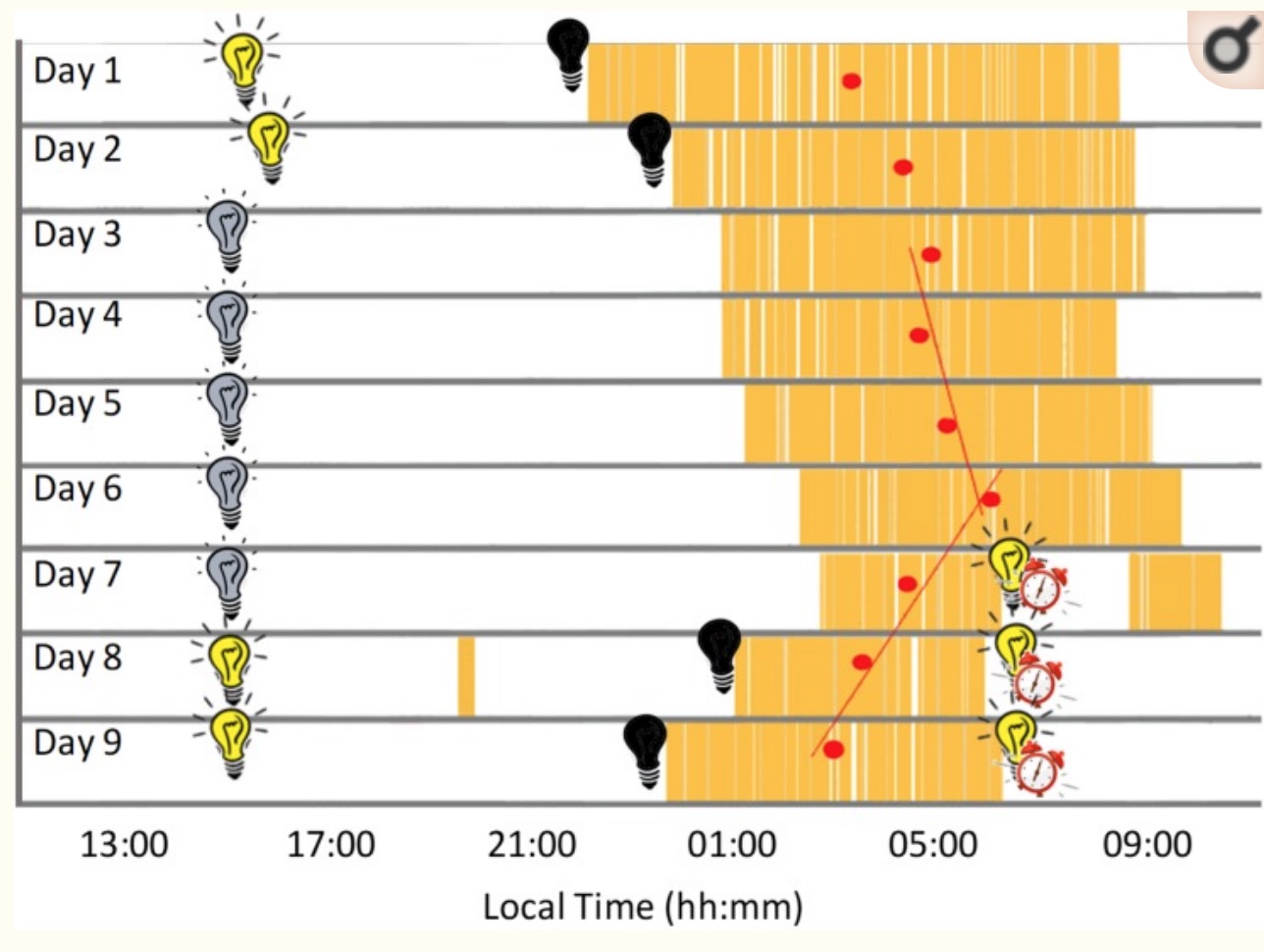
While light is the main way the body resets the circadian clock, food timing and temperature changes can also make an impact.
What about melatonin?
Often referred to as the ‘sleep hormone’, melatonin levels rise at night when it is dark. It is a key molecule in circadian rhythm signaling.
Animal studies specifically show that melatonin is part of regulating some of the core clock genes. In fact, without melatonin, the PER1 gene doesn’t rise and fall rhythmically.[ref]
Melatonin is produced by the pineal gland in large amounts at night due to the lack of light. Exposure to artificial light at night significantly decreases overnight melatonin levels.
During the day, melatonin levels should be low – decreased by the bright light. In people with depression, research shows that melatonin levels are often higher than they should be during the day. This is possibly due to indoor lighting not suppressing the melatonin levels enough in an individual (hyposensitivity due to the low light).[ref]
When Rhythms Go Awry
Disrupted circadian rhythms have far-reaching implications, increasing risks for diabetes, cardiovascular diseases, certain cancers, mood disorders, and obesity. The burgeoning volume of research in recent years has been remarkable. This has prompted the IARC (International Agency for Research on Cancer, part of the WHO) to classify chronic exposure to light at night as a potential carcinogen since 2007.
Circadian rhythms are the natural biological rhythms that shape our biology. Most people know about the master clock in our brain that keeps us on a wake-sleep cycle over 24 hours. This is driven by our master ‘clock’ genes.
Quick Recap: Circadian rhythms are foundational to optimal health. Morning sunlight sets the rhythm, while evening blue light can disrupt it. Understanding and respecting these natural cycles, along with recognizing the role of genetic differences, is vital for maintaining health and preventing disease.
Genotype Report: Clock Genes
Not a member? Join here. Membership lets you see your data right in each article and also gives you access to the member’s only information in the Lifehacks sections.
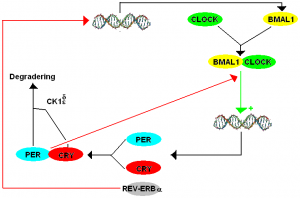 The CLOCK and BMAL1 (ARNTL) genes are the core clock genes that target and turn on other genes. They are regulated and turned off by rising levels of the cryptochromes, CRY1 and CRY2 and the period genes PER1, PER2, and PER3.[ref] This forms the daily feedback loop of rising CLOCK and BMAL1 that are then inhibited by rising levels of CRY and PER, giving a 24-hour rhythm.
The CLOCK and BMAL1 (ARNTL) genes are the core clock genes that target and turn on other genes. They are regulated and turned off by rising levels of the cryptochromes, CRY1 and CRY2 and the period genes PER1, PER2, and PER3.[ref] This forms the daily feedback loop of rising CLOCK and BMAL1 that are then inhibited by rising levels of CRY and PER, giving a 24-hour rhythm.
CLOCK gene variants:
The most well-studied CLOCK variant, with a large impact on circadian rhythm, is the first one, rs1801260.
Check your genetic data for rs1801260 3111T/C (23andMe v4, v5, AncestryDNA):
- G/G: higher activity in the evening, delayed sleep onset, and less overall sleep time on average.[ref][ref] higher BMI, slower gastric motility[ref][ref]; higher ghrelin (hunger hormone) levels[ref][ref][ref]; more manic episodes in bipolar[ref]
- A/G: somewhat higher evening activity and slightly later bedtime; higher BMI; slower gastric motility; higher ghrelin
- A/A: typical
Members: Your genotype for rs1801260 is —.
Check your genetic data for rs11932595 (23andMe v4, v5, AncestryDNA):
- G/G: increased relative risk of male infertility, shorter sleep[ref][ref][ref] higher fasting glucose[ref]
- A/G: increased relative risk of male infertility, shorter sleep
- A/A: typical, longer sleep duration[ref]
Members: Your genotype for rs11932595 is —.
Check your genetic data for rs4864548 (23andMe v4, AncestryDNA):
Members: Your genotype for rs4864548 is —.
ARNTL gene (BMAL1):
BMAL1 binds with CLOCK to increase the production of the PER and CRY circadian genes.
Check your genetic data for rs3789327 (23andMe v4; AncestryDNA):
- A/A: typical
- A/G: lower incidence of myocardial infarction
- G/G: lower incidence of myocardial infarction [ref]
Check your genetic data for rs3816358 (23andMe v4, AncestryDNA):
- A/A: reduced relative risk of breast cancer compared to other shift workers[ref]
- A/C: reduced relative risk of breast cancer compared to other shift workers
- C/C: typical risk of breast cancer in shift workers
Members: Your genotype for rs3816358 is —.
Check your genetic data for rs7950226 (23andMe v4, AncestryDNA):
- A/A: increased risk of diabetes and gestational diabetes[ref] increased risk of prostate cancer[ref]
- A/G: increased risk of gestational diabetes; increased risk of prostate cancer
- G/G: typical
Members: Your genotype for rs7950226 is —.
Check your genetic data for rs6486122 (23andMe v4, v5; AncestryDNA):
- T/T: increased risk of heart disease, diabetes[ref]
- C/T: slightly increased risk of heart disease, diabetes
- C/C: typical
Members: Your genotype for rs6486122 is —.
Check your genetic data for rs11022775 (23andMe v4; AncestryDNA):
Members: Your genotype for rs11022775 is —.
Check your genetic data for rs969485 (23andMe v4, v5; AncestryDNA):
- G/G: increased risk of breast cancer with night shift work[ref]
- A/G: increased risk of breast cancer with night shift work (64%)
- A/A: typical
Members: Your genotype for rs969485 is —.
Check your genetic data for rs2278749 (23andMe v4, v5; AncestryDNA):
- T/T: night shift work less likely to increase breast cancer risk[ref], more fertile[ref]
- C/T: night shift work less likely to increase breast cancer risk[ref]
- C/C: typical
Members: Your genotype for rs2278749 is —.
PER1 gene:
PER1 codes for the ‘period circadian protein homolog 1’ protein which, along with CRY (below), is the other half of the core genes involved in our circadian rhythm.
A study in 2012 found that a variant, rs7221412, altered the natural timing of activity. Those with the A/A genotype may naturally wake up about an hour earlier than those with G/G, while A/G falls in the middle.
Check your genetic data for rs7221412 (23andMe v4):
- A/A: one hour earlier peak in circadian rhythm, more like to wake earlier[ref]
- A/G: peak was midway between A/A and G/G
- G/G: one hour later peak in circadian rhythm, more likely to wake later
Members: Your genotype for rs7221412 is —.
PER2 gene:
A recent study looked at the expression of clock genes after weight loss and found that PER2 expression increased after weight loss.[ref]
Check your genetic data for rs2304672 (23andMe v4):
- C/C: associated with abdominal obesity, snacking, stress with dieting[ref]; higher lipids[ref]
- C/G: associated with abdominal obesity, snacking, stress with dieting, higher lipid levels
- G/G: typical (23andme orientation)
Members: Your genotype for rs2304672 is —.
CRY2 gene:
A study of obese and lean women tracked their clock gene expressions in fat tissue over a 24-hour period. The study found CRY2 and REV-ERB ALPHA up-regulate in obesity over a 24-hour period.[ref]
Check your genetic data for rs7123390 (23andme v4, AncerstyDNA):
- postmenopausal women with A/A had half the risk of estrogen and progesterone receptor-negative breast cancer.[ref]
NR1D1 (REV-ERB Alpha)
Check your genetic data for rs2314339 (23andMe v4, v5, AncestryDNA): (T is the minor allele)
- REV-ERB alpha has associations with both a circadian mechanism and the biological action of lithium carbonate. For bipolar disorder, those with at least one copy of the T allele were 3.5x less likely to improve on lithium carbonate therapy.[ref]
- Minor allele (T) carriers are less likely to be abdominally obese.[ref]
Check your genetic data for rs2071427 (23andMe v4, v5, AncestryDNA): (T is the minor allele)
- The minor allele, T, has associations with a higher risk of obesity and higher BMI.[ref]
Other genes:
FTO is a gene with variants that are associated with an increased risk of obesity (nicknamed the fatso gene). A 2015 study found that FTO-deficient mice had robust circadian locomotor activity rhythms, while “Overexpression of FTO represses the transcriptional activation by CLOCK and BMAL1.”[ref] Other studies have shown that over-expression of FTO leads to increased body fat.[ref]
Lifehacks: Optimizing Circadian Rhythm
The rest of this article is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
References:
Updated and revised Dec 2017, Aug. 2018